Batch and Global Analysis of Fluorescence Lifetimes
Key Points
- Large datasets of fluorescence lifetimes can be processed in Edinburgh Instruments’ FAST software.
- Fluorescence lifetime datasets of a three-fluorophore mixture (erythrosin b, fluorescein, and 9-aminoacridine) were processed in FAST using Batch Processing and Global Analysis, providing valuable insights into the sample’s lifetime components and their relative weight distribution.
- In Batch Analysis, the fluorescence lifetimes of the mixture were automatically processed in a single batch, and the decays were analysed independently.
- In Global Analysis, the fluorescence lifetimes of the mixture were linked, and the same global lifetime component was used to fit all the decays in the dataset.
Introduction
Analysing large fluorescence lifetime datasets requires automated processing and the identification of common patterns across the dataset. Batch Analysis and Global Analysis are two automated processing methods for fluorescence lifetime datasets that are available in Edinburgh Instruments FAST lifetime analysis software. In this Application Note, fluorescence lifetimes and relative weights of a three-fluorophore mixture of erythrosin b, fluorescein, and 9-aminoacridine (9AA) were analysed using both batch and global analysis in FAST, and the processing methods compared.
Methods and Experimental Setup
The fluorescence lifetimes were acquired using an Edinburgh Instruments FLS1000 Photoluminescence Spectrometer equipped with a PR2 Microwell Plate Reader and a High-Speed Hybrid Photodetector (HS-HPD). An EPL-445 picosecond pulsed diode laser was used as the excitation source and operated at 10 MHz, and the lifetimes were acquired using TCSPC. Each fluorophore was diluted in methanol to give an absorbance of around 0.03 for erythrosin b, 0.06 for fluorescein, and 0.09 for 9AA at the EPL’s operational wavelength, 437 nm. Three wells were filled with ~150 μl of each pure fluorophore, and ten wells were filled with ~150 μl of the three-fluorophore mixture. Representative figures of the plate reader and the plate reader interface in Fluoracle® that shows the ten selected wells containing the mixture can be seen in Figures 1a and 1b, respectively.
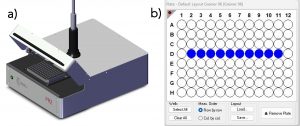 Figure 1. a) The FLS1000 plate reader (PR2), and b) PR2 Plate Reader interface in Fluoracle
Figure 1. a) The FLS1000 plate reader (PR2), and b) PR2 Plate Reader interface in Fluoracle
Lifetime Analysis of the Individual Decays
The fluorescence lifetimes of the three pure fluorophores can be seen in Figure 2. Erythrosin b (Figure 2a) exhibits the shortest decay (0.46 ns), and therefore, the fit was a deconvolution of the instrument response function (IRF) and the exponential decay of the sample. The IRF was not considered for Fluorescein and 9AA as they were characterised by longer lifetimes. Figures 2b and 2c show the Fluorescein (4.41 ns) and 9AA’s (12.41 ns) decays, fitted by a single exponential decay.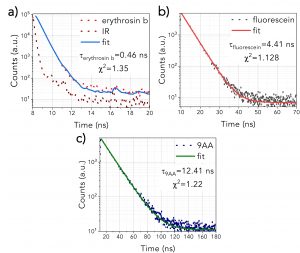
Figure 2. Fluorescence lifetimes of a) erythrosin b, b) fluorescein, c) 9AA
Batch Analysis of the Mixture
The ten fluorescence decays of the mixture were first analysed using batch analysis. In batch analysis multiple decays can be automatically analysed in a single batch. The decays are analysed independently and fitted to a chosen model to obtain independent information for each decay. The ten decays of the mixture were imported into FAST, and batch analysed using the Exponential Components Analysis routine. A three-component exponential model was chosen since the mixture comprises three fluorophores (Figure 3). In the case of an unknown sample, the user could start fitting with a single exponential component and then add additional components if required after reviewing the residuals and chi-squared optimisation.
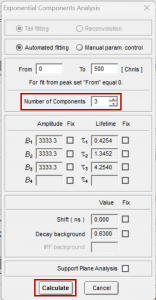
Figure 3. Parameters for batch processing in FAST
FAST then performs fitting for each fluorescence decay providing numerical information about τ1, τ2, and τ3, their amplitudes, Bi, (with amplitude standard deviation, ΔBi) and weights, fi (%) (with weights standard deviations, Δfi %). The distributions of the relative weights, fi (%), of each exponential component are plotted in Figure 4 alongside the average lifetime of each component. The average lifetimes of the τ1, τ2 and τ3 components closely match those of pure erythrosin B, fluorescein and 9AA, respectively.
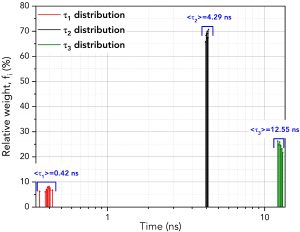
Figure 4. Distribution of the lifetimes and relative weights of the three components across the ten decays using batch analysis
Global Analysis of the Mixture
In global analysis, the component lifetimes are no longer independent across the multiple decays in the dataset; they are a global parameter. Global analysis assumes that a global component lifetime can describe all the fluorescence decays in a dataset.
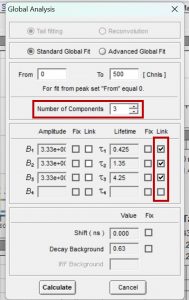
Figure 5. Parameters for global analysis in FAST
Figure 5 shows how to set the parameters in FAST to perform global analysis. τ1, τ2 and τ3 are set to be linked. Linking a lifetime component means that the same component lifetime is a global parameter and is used to fit all decays in the dataset. The component’s lifetime is then optimised across all decays in the dataset during the fitting routine to find the lifetime that best describes all decays collectively. The lifetimes and relative weight of the global analysis results are shown graphically in Figure 6. Since the lifetimes are now a global parameter, there is no longer a distribution for each lifetime component. Instead, there is a single lifetime value for each component that best describes all ten decays.
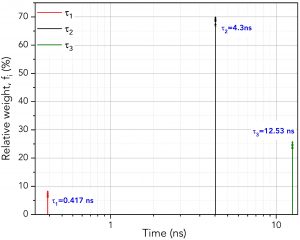
Figure 6. Lifetimes and relative weights of the three components using global analysis across the ten decays
It is worth mentioning that if τ1, τ2, and τ3 were all set to unlinked, then the results would precisely match the batch processing analysis of the previous section. Thus, the user can perform both techniques promptly by linking and unlinking the time variables.
Conclusion
This Application Note outlined the successful application of Edinburgh Instruments’ FAST software for batch analysis and global analysis in studying the lifetimes of a three-fluorophore mixture. The batch analysis approach treats the lifetimes of each component in the mixture as independent parameters, while the global analysis approach considers them as linked (global parameters). Both methods offer valuable insights into the lifetimes and relative weight distribution of the individual components within a multi-fluorophore mixture. The choice between these methods should be based on the specific samples being studied and their intended applications.









