NIR Emission Spectra of PbS Quantum Dots Using the FS5 Spectrofluorometer
Key Points
- The FS5 Spectrofluorometer with an NIR InGaAs detector can measure emission spectra up to 1650 nm.
- Absorption and emission spectra of PbS quantum dots are measured using the FS5.
- Solvent absorption in the NIR can distort emission spectra, making appropriate solvent choice highly important.
Introduction
Near infrared emitting (NIR) semiconductor quantum dots (QDs) possess highly tuneable properties, and high photoluminescence quantum yields. In biological imaging, it is highly advantageous to synthesise probes with absorption and emission wavelengths in the NIR region. Firstly, this will prevent damage to biological tissues, which is a fundamental drawback of UV region fluorophores. Secondly, these wavelengths have increased tissue penetration, which is ideal for surgical applications, and finally show increased spatial resolution.1,2
One commercially available series of NIR-emitting QDs are lead sulfide (PbS) QDs. Their tuneable emission characteristics are controlled by capping with amphiphilic molecules, which aid the stabilisation of small-sized nanoparticles, preventing uncontrolled growth.3 To characterise these useful materials, QDs are often studied in solution, where they must be dispersed. QDs are often designed to be hydrophobic, requiring the use of an organic solvent for dispersion. However, the NIR absorption of organic solvents can interfere with the emission of the sample. In this application note, the photoluminescence spectra of NIR PbS quantum dots are measured in three organic solvents using the FS5 Spectrometer equipped with an NIR InGaAs detector.
Materials & Methods
PbS QDs were acquired from Quantum Solutions. Dispersions were prepared in tetrachloroethylene (PCE) (anhydrous, Sigma), dichloromethane (DCM) (Uvasol®, Merck), and toluene (anhydrous, Sigma) with an absorbance of ~0.1 at the excitation wavelength of 670 nm. Samples were loaded into 10 mm pathlength 4-sided cuvettes. The DS5 UV-Vis Spectrophotometer was used for absorbance measurements. For emission spectra, measurements were made using the FS5 Spectrofluorometer (Figure 1) equipped with a
150 W Xe arc lamp and a InGaAs-1650 NIR detector (NIRA+ upgrade). Cuvettes were held in the SC-05 Standard Cuvette Holder Module.

Figure 1. Edinburgh Instruments FS5 Spectrofluorometer.
Absorption & Emission Spectra of PbS
The absorption and emission of PbS QDs were measured using the DS5 and FS5. The DS5 has an extended range absorption range to 1100 nm, allowing the NIR absorption peak of PbS in PCE to be recorded (Figure 2).
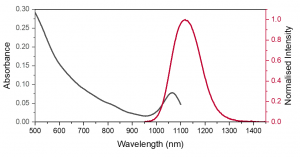
Figure 2. Absorption spectrum of PbS QDs in PCE measured on the DS5 from 500-1100 nm (black). Normalised emission spectrum of PbS QDs in PCE (red), excited at 670 nm.
The absorption of PbS in PCE shows two important features, a broad visible absorption band up to 950 nm and narrow NIR absorption band centred at 1050 nm. This longer wavelength absorption band overlaps with the emission band of PbS QDs in PCE. The small Stokes shift between the absorption and emission NIR peaks results in a high degree of spectral overlap shown in Figure 2. This could cause reabsorption which reduces the emission on the rising edge of the spectra and resulting in spectral distortion. It is therefore important to keep the concentration low enough to minimise this effect, which is a common feature in NIR emitters.
Influence of the Solvent on the PbS Emission Spectrum
QDs may be dissolved in a range of organic solvents. To investigate the influence of the solvent on the emission spectra, the same QDs were suspended in DCM and toluene. The resulting normalised spectra are shown in Figure 3 and highlight the importance of solvent choice when measuring emission spectra in the NIR.
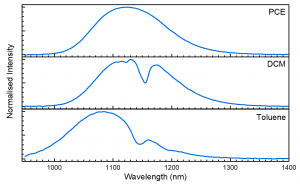
Figure 3. Emission spectra of PbS QDs in PCE (top), DCM (middle), and toluene (bottom) excited at 670 nm.
The emission peak is shifted by solvent interactions, with toluene shifted to shorter wavelengths than PCE and DCM. However, the more extreme effect is that of the troughs within the emission peak caused by direct absorption of the PbS emission by the toluene and DCM solvent molecules.
Many common solvents have absorption bands that can interfere with the sample emission profile in the NIR. This makes checking the properties of the solvent especially important when collecting emissions in the NIR region.4
Conclusion
The FS5 Spectrofluorometer can measure emission spectra up to 1650 nm when equipped with the InGaAs-1650 NIR detector (NIRA+ upgrade). It has been shown that common solvents such as toluene and DCM have absorption bands above 1000 nm, which can obstruct the emission spectrum of the NIR-emitting sample. These solvent effects make further measurements such as quantum yield challenging in as the solvent absorption must be considered for an accurate quantum yield value.
References
- Imamura, S. Yamada, S. Tsuboi, Y. Nakane, Y. Tsukasaki, A. Komatsuzaki and T. Jin, Near-infrared emitting PbS quantum dots for in vivo fluorescence imaging of the thrombotic state in septic mouse brain, Molecules, 2016, 21, 1-12.
- Asha Krishnan, K. Yadav, P. Roach and V. Chelvam, A targeted near-infrared nanoprobe for deep-tissue penetration and imaging of prostate cancer, Biomater Sci, 2021, 9, 2295–2312.
- Gulati, M. Sachdeva and K. K. Bhasin, in AIP Conference Proceedings, AIP, 2018, p. 030214.
- Hoshi, K. Suzuki, N. Hasebe, T. Yoshihara and S. Tobita, Absolute Quantum Yield Measurements of Near-Infrared Emission with Correction for Solvent Absorption, Anal Chem, 2020, 92, 607–611.









