Study of Fluorescence Quenching Kinetics Using Stopped-Flow
Introduction
The understanding of reaction kinetics is an important area of study across biology and chemistry. Through the study of reaction kinetics the reaction rate, reaction order and the underlying molecular mechanisms can be determined. A powerful method for determining the kinetics of a reaction is by monitoring the concentration of the reactants or products over time using absorption or fluorescence spectroscopy. For slow reactions, the reactants can simply be mixed by hand or using a magnetic stirrer. However, for fast reactions, where the lifetime of the reaction is comparable with the mixing time, more specialised techniques must be used such as stopped-flow.

Figure 1: FLS1000 Photoluminescence Spectrometer with Stopped-Flow Accessory.
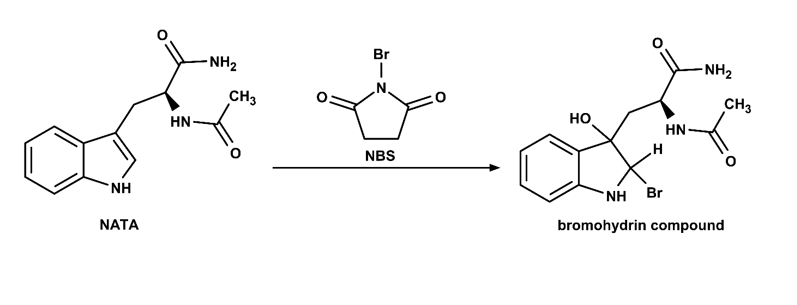
In stopped-flow the reactants are quickly forced into a cuvette where they rapidly mix and the change in absorbance or fluorescence intensity monitored over time. Edinburgh Instruments offers a stopped-flow accessory for both the FLS1000 and the FS5 Fluorescence Spectrometers, extending the capability of these systems for the measurement of rapid reaction kinetics. A model system for studying reaction kinetics is the fluorescence quenching of N-acetyltryptophanamide (NATA) by N-bromosuccinimide (NBS).¹² NATA is oxidised by NBS to form a non-fluorescent bromohydrin, as shown in Figure 2, and the kinetics of the reaction can therefore be investigated by monitoring the change in fluorescence intensity of NATA as a function of time.³ By performing the reaction with an excess of NBS, the reaction exhibits pseudo-first order kinetics with a rate constant that is proportional to the concentration of NATA. At the concentrations used here this reaction is too fast to be monitored using manual mixing and therefore serves as a useful verification test of the stopped-flow accessory.¹
Figure 2: Oxidation of N-acetyl tryptophanamide (NATA) by N-bromosuccinimide (NBS).
Methods and Materials
N-acetyltryptophanamide and N-bromosuccinimide were dissolved in phosphate buffered saline (PBS) at pH 7.3. Three NATA solutions were prepared with concentrations of 5 µM, 10 µM and 20 µM. The NBS quencher was kept in excess with concentrations of 50 µM, 100 µM and 200 µM respectively. The absorption spectrum of NATA was recorded using the transmission detector of the FS5 Spectrofluorometer with an excitation bandwidth of 3 nm. Fluorescence measurements were performed using the FLS1000 Photoluminescence Spectrometer equipped with double excitation and emission monochromators, photomultiplier tube detector (PMT-980), a 450 W Xe lamp and the N-M04MM stopped-flow accessory. The emission spectrum of NATA was recorded at an excitation wavelength of 280 nm using excitation and emission bandwidths of 3 nm and 1.5 nm respectively. For fluorescence kinetic measurements the NATA and NBS solutions were loaded into the A & B syringes of the N-M04MM stopped-flow accessory. Syringe C was filled with PBS buffer to serve as a reference. The TTL output trigger of the FLS1000 was used to initiate the N-M04MM injection and the total stop volume was set to 400 µl. The kinetic spectra were recorded using an excitation wavelength of 280 nm with a 5 nm bandpass, an emission wavelength of 360 nm with a 5 nm bandpass, and a temporal resolution of 0.2 ms.
Results-Discussion
The absorption and emission spectra of NATA solution were recorded and shown in Figure 3. NATA has an absorption maximum at 280 nm and an emission maximum close to 360 nm and these were therefore chosen as the excitation and emission wavelengths for the subsequent fluorescence kinetic measurements.
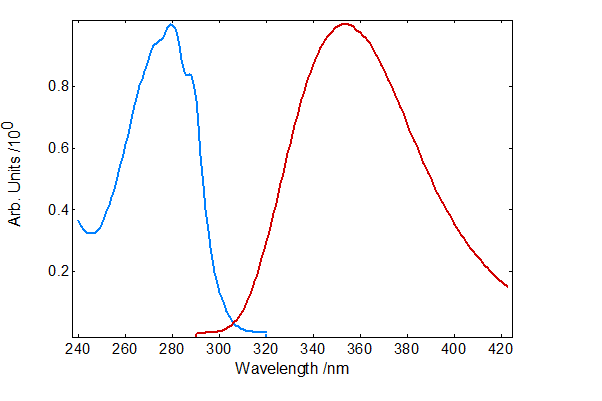
Figure 3: Absorption (blue) and emission (red) spectra of NATA in PBS buffer.
A fluorescence kinetic of the quenching of 10 µM NATA by 100 µM NBS is shown in Figure 4 (red). The fluorescence quenching kinetic was analysed using the FLS1000 Fluoracle operating software. The data was fit well using a single monoexponential decay with a time constant of 28 ms. The fluorescence kinetic was reorded with a high temporal resolution of 0.2 ms which greatly improves the quality of the recorded kinetic and enables accurate fitting of the decay and relaible rate constants to be extracted. The FLS1000 offers flexible time ranges and resolutions for monitoring kinetics; equally capable of measuring fast decays with temporal resolutions of <0.1 ms and monitoring slower kinetics over the course of hours. A fluorescence kinetic following the injection of 10 µM NATA and PBS buffer was also measured as a reference and is shown in blue. It can be seen that in the absence of NBS the fluorescence remains constant which confirms that the decay observed when NATA and NBS are injected must be fluorescence quenching and not caused by a change in absorbance of the solutions or an artefact in the system. The difference in fluorescence intensity between the start of the red decay and the blue baseline is due to the dead time of the stopped-flow system; the time between solution mixing and the first measurement. The dead time can be calculated from the intercept of the exponential fit and the baseline,1 and is approximately 12 ms.
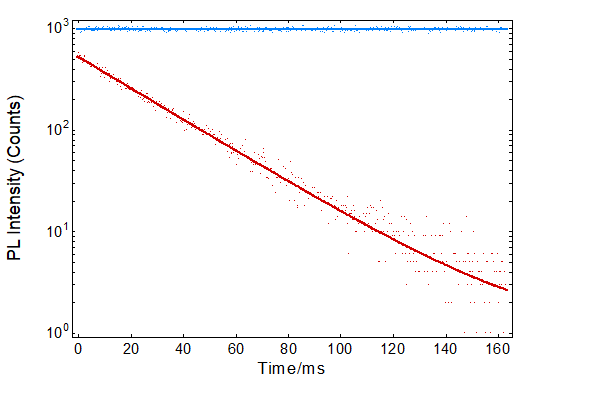
Figure 4: Fluorescence kinetics after the injection of 10 µM NATA and 100 µM NBS (red) and after the injection of 10 µM NATA and PBS buffer (blue). Each kinetic trace (dots) is the superposition of 5 consecutive injections and acquisitions. The traces were fitted with a monoexponential fit (red) and a linear fit (blue) using Fluoracle.
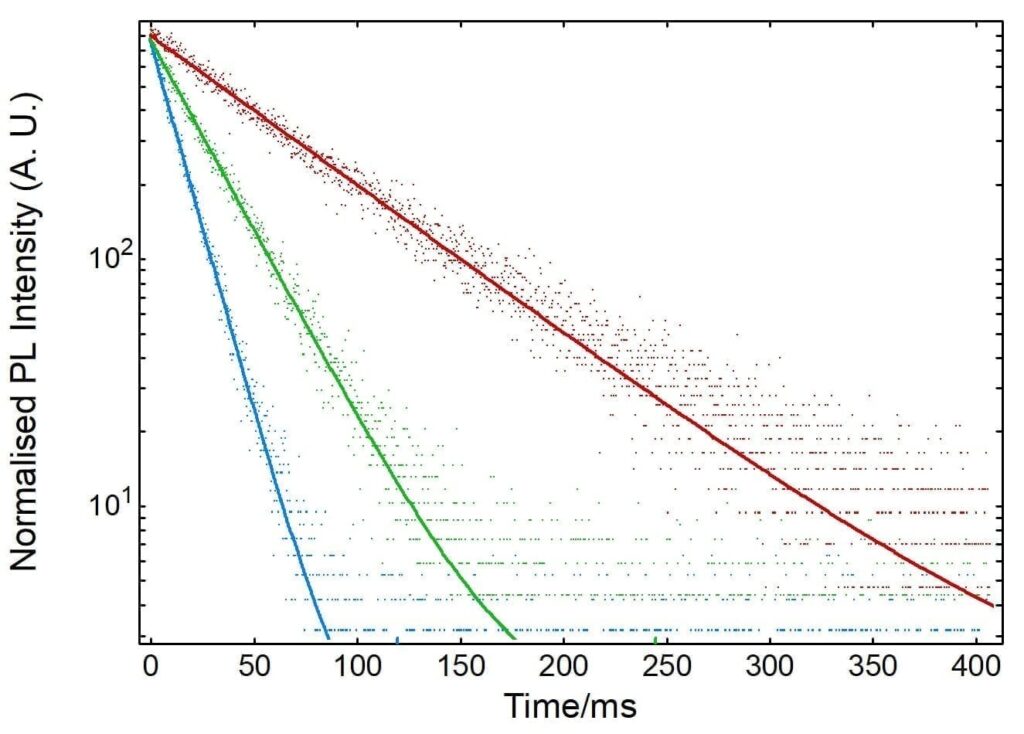
The influence of NATA concentration on the rate of fluorescence quenching was also investigated. The fluorescence quenching kinetics of NATA with concentrations of 5 µM, 10 µM and 20 µM were measured and fit using Fluoracle with monoexponential decays as shown in Figure 5. As the concentration of NATA is increased the time constant of the decay decreases indicating that the reaction proceeds more rapidly, as is expected for first order reaction kinetics. From the decay time constants the first order reaction rates can be calculated and are given in Table 1.
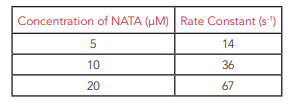
Table 1: Variation of first order rate constant with NATA concentration
Figure 5: Normalised fluorescence kinetics of NATA quenching by NBS at different concentrations. Solid lines are monoexponential fits using
Fluoracle.
Conclusion
The fluorescence quenching kinetics of N-acetyltryp-tophanamide with N-bromosuccinimide were measured using the FLS1000 with stopped-flow accessory. The fluorescence quenching kinetics were fit using the FLS1000’s versatile Fluoracle operating software and the first order rate constants of the reaction determined. This application note demonstrates that the FLS1000 combined with the stopped-flow accessory is a powerful tool for measuring rapid reaction kinetics. The flexible time ranges and temporal resolutions offered by the FLS1000 for measuring kinetics makes it ideally suited for measuring both fast decays with resolutions of <0.1 ms and the monitoring of slower kinetics over many hours.
References
- B. F. Peterman, Measurement of the Dead Time of a Fluorescence Stopped-Flow Instrument, Anal. Biochem. 93, 442-444 (1979)
- John J. Correia and H. William Detrich, III Biophysical Tools for Biologists Volume One: In Vitro Techniques, Academic Press, USA, 457 (2008)
- B. F. Peterman and K. J. Laidler, The Reactivity of Tryptophan Residues in Proteins Stopped-Flow Kinetics of Fluorescence Quenching, Biochem. Biophys. Acta 577, 314-323 (1979)
Keep In Touch
If you have enjoyed reading our Stopped Flow Application Note, why note sign-up to our newsletter via our red Sign-up button below.