Multiphoton Imaging of Mouse Intestine
Introduction
Two-photon excited fluorescence (2PEF) and second harmonic generation (SHG) are complementary multiphoton imaging techniques for studying biological samples. Both imaging techniques utilise femtosecond pulsed infrared excitation light to generate shorter wavelength light to image the sample but operate via fundamentally different physical processes (Figure 1). In 2PEF, two infrared photons are simultaneously absorbed by a fluorophore promoting it to an excited state which then radiatively relaxes emitting shorter wavelength fluorescence. In contrast, SHG is not an absorption and emission process and instead the two infrared photons combine in a non-linear optical material with a particular symmetry to generate a new photon with exactly half the wavelength of the incident photons. Both techniques take advantage of the lower scattering and absorption of infrared light to enable imaging deep into tissue. In this application note, an Edinburgh Instruments RMS1000 Confocal Raman Microscope is used to image a tissue section of mouse intestine using 2PEF and SHG microscopy.
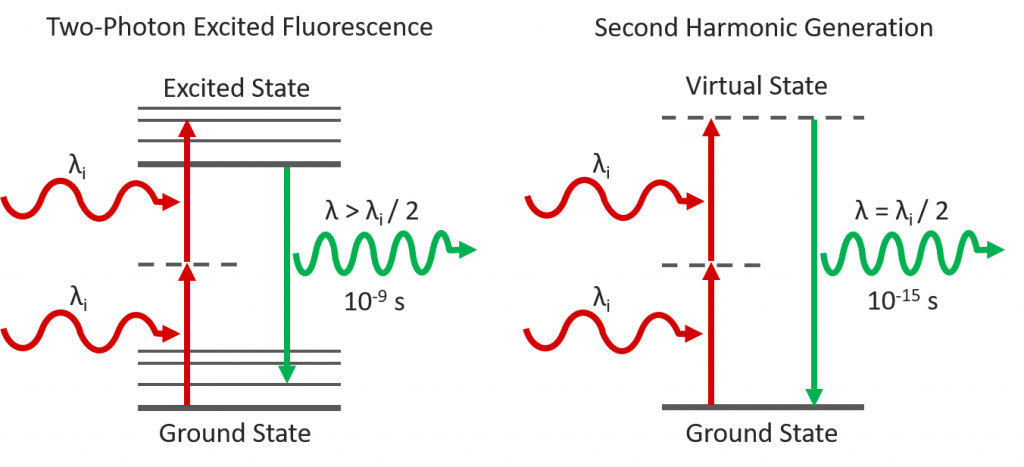
Figure 1: Two-Photon Excited Fluorescence vs Second Harmonic Generation.
Experimental Configuration
The sample to be imaged was a section of mouse intestine tissue stained with Alexa Fluor® 568. The RMS1000 was equipped with a motorised XYZ stage and a 40x NA = 0.75 objective. For spectral imaging, the RMS1000 was equipped with a back-illuminated CCD camera and for lifetime imaging; a photon counting Hybrid Photodetector and time-correlated single photon counting (TCSPC) electronics. 2PEF and SHG both require a very high excitation intensity, which is achieved using a mode-locked femtosecond pulsed laser. The RMS1000 has external laser coupling ports that enable the optical coupling of femtosecond lasers into the microscope. The optical setup for the femtosecond excitation source is shown in Figure 2. The laser was a Chromacity 1040 HP femtosecond fibre laser with an output wavelength of 1040 nm and an 80 MHz repetition rate (Chromacity Ltd., UK). For lifetime imaging the output of the laser was pulse picked to the desired pulse frequency using a pulseSelect pulse picker (APE GmbH, Germany). A small fraction of the pulse picker output is picked-off into an Edinburgh Instruments OT900 optical trigger module to trigger the TCSPC electronics. For spectral measurements, the pulse picker is bypassed, and the 80 MHz laser output is coupled directly into the RMS1000.
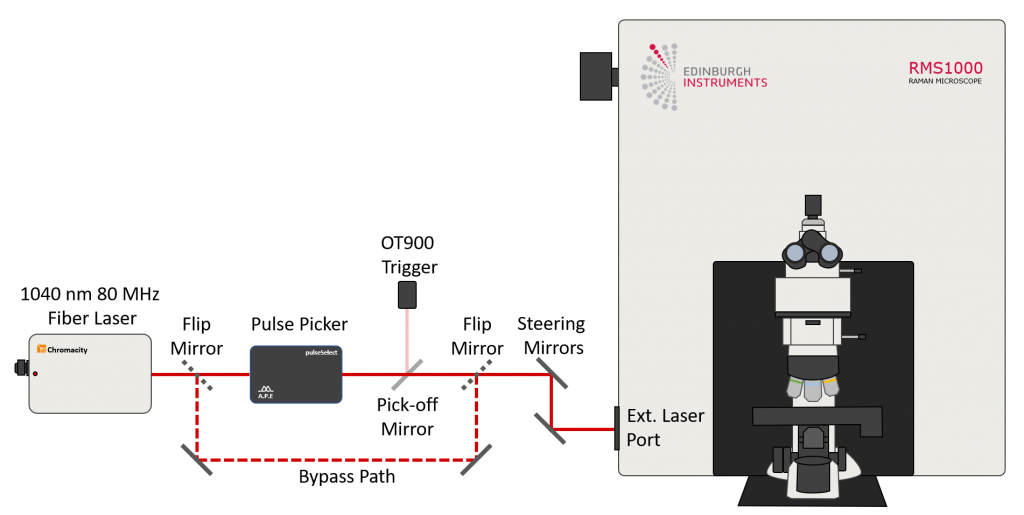
Figure 2: Optical setup for 2PEF and SHG microscopy with the Edinburgh Instruments RMS1000.
2PEF & SHG Spectral Imaging with CCD Camera
The intestine tissue section was first imaged spectrally using the CCD camera of the RMS1000. A 900 μm x 800 μm area of the sample was mapped with a spatial resolution of 2 μm. The sample was excited at 1040 nm 80 MHz and the 2PEF and SHG signals acquired simultaneously using the CCD camera. The resulting multiphoton image is shown in Figure 3a. 2PEF at 630 nm from the Alexa Fluor® 568 dye is shown in teal and reveals the structure of the intestinal villi. The area shown in pink is SHG at 520 nm from fibrillar collagen near the intestinal wall. SHG only occurs from molecular structures that are non-centrosymmetric and fibrillar collagen is a common biological structure with this property, eliciting a strong SHG response. Each point in the image has a corresponding spectrum, and the spectra at two points A and B are shown in Figure 3b. At point A there is only 2PEF from the Alexa Fluor® 568 dye which has a broad emission centred at 630 nm, while at point B there is an additional sharp peak at 520 nm which is the SHG signal. The colour image in Figure 3a was obtained by plotting the intensity of the spectra at 630 nm and 520 nm for 2PEF and SHG respectively.
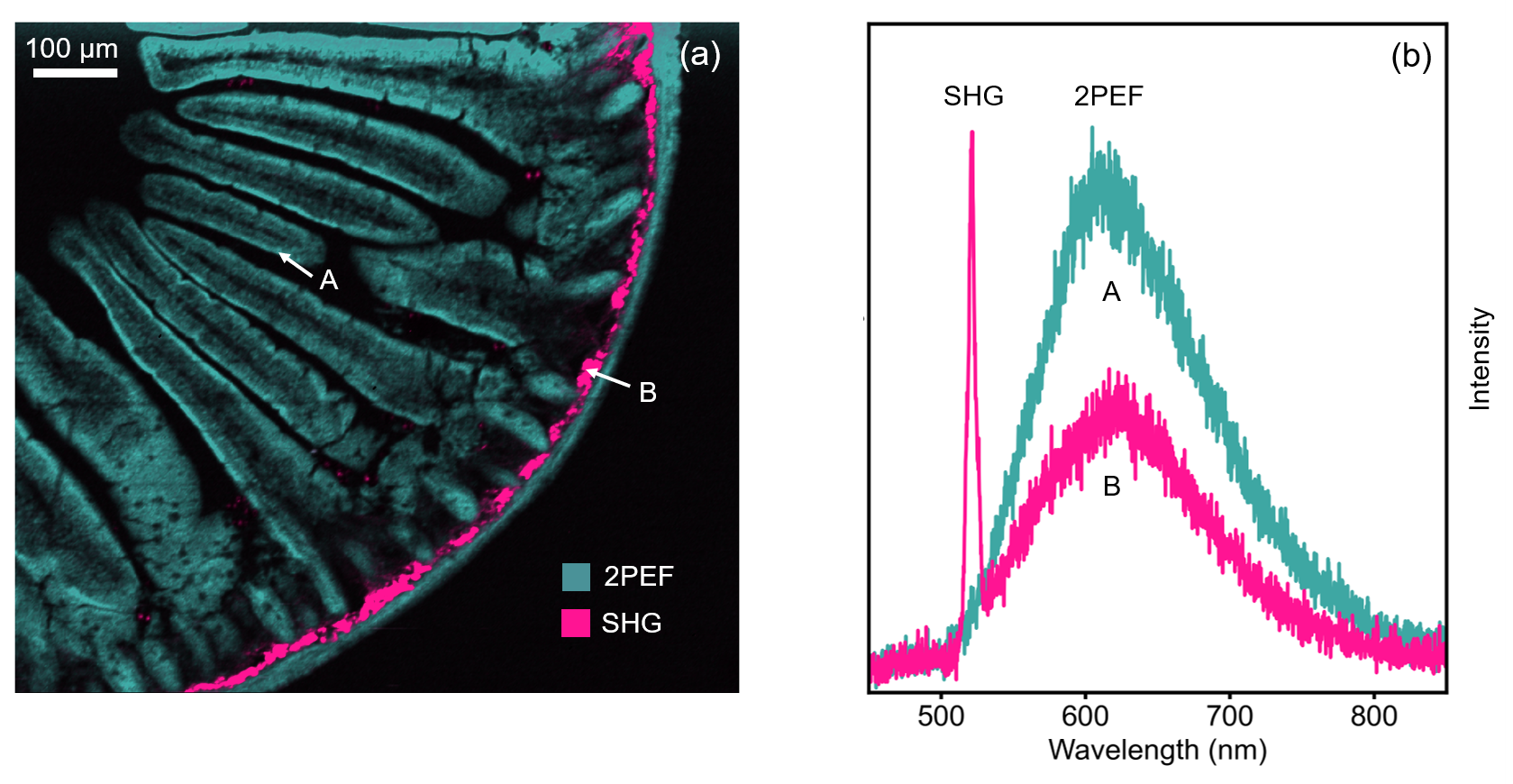
Figure 3: (a) 2PEF and SHG image of mouse intestine section stained with Alexa Fluor® 568 and (b) extracted spectra from two points in the image.
2PEF Lifetime Imaging with Hybrid Photodetector
Additional information can be obtained by two-photon fluorescence lifetime imaging. For lifetime imaging the repetition rate of the laser was lowered to 20 MHz using the pulse picker to ensure complete fluorescence decay between pulses. The same 900 μm x 800 μm area was mapped and the fluorescence decay at each point recorded using TCSPC on the photon counting Hybrid Photodetector. Each fluorescence decay was fit with an exponential model using the RMS1000 Ramacle® software and the resulting lifetime image is shown in Figure 4a. The lifetime image shows a decreased fluorescence lifetime in the intestinal crypts near the intestinal wall compared with the villi; an example of the increased information that can routinely be obtained from lifetime imaging.
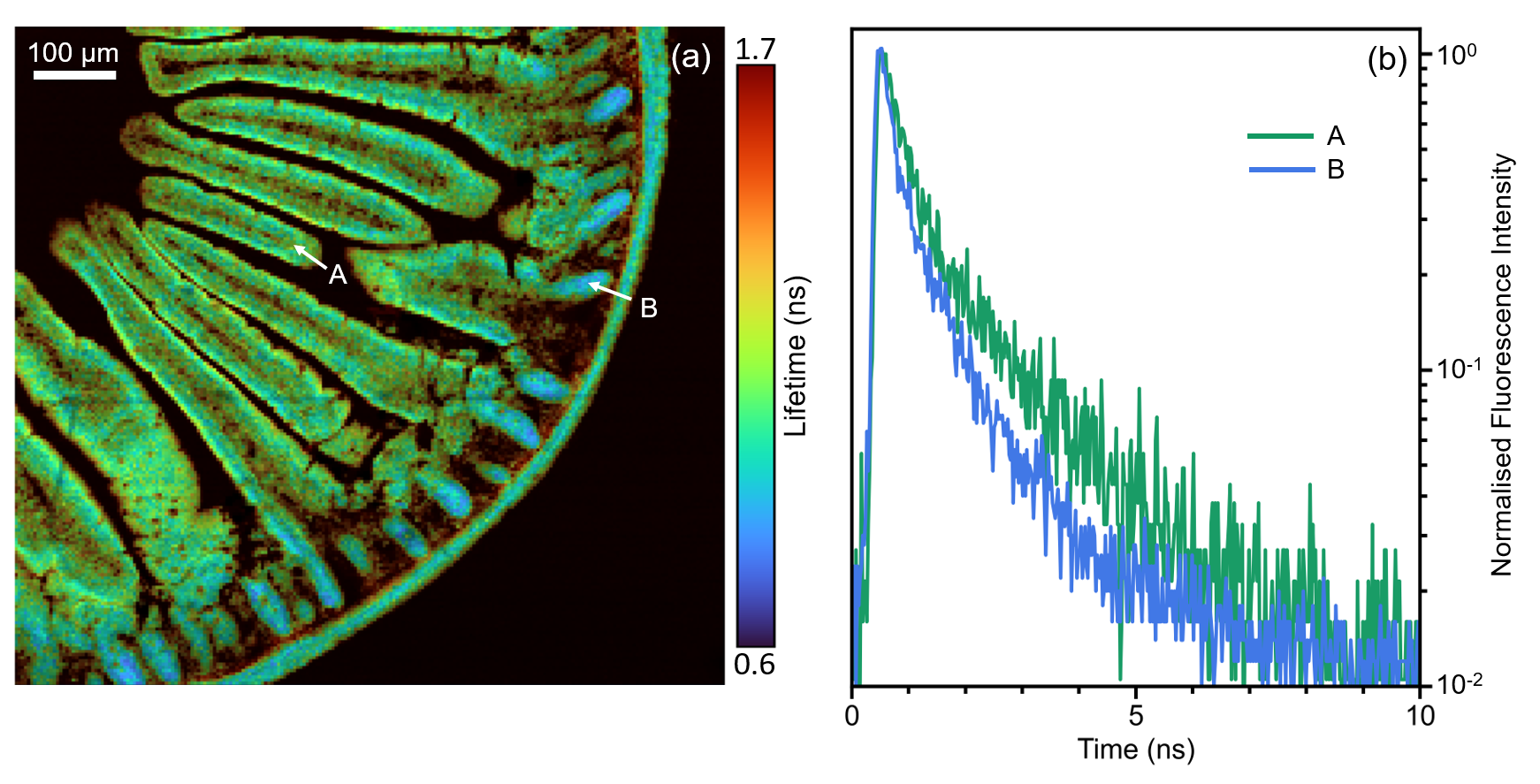 Figure 4: (a) 2PEF lifetime image of mouse intestine section stained with Alexa Fluor® 568 and (b) extracted fluorescence decays from two points in the image.
Figure 4: (a) 2PEF lifetime image of mouse intestine section stained with Alexa Fluor® 568 and (b) extracted fluorescence decays from two points in the image.
Conclusion
A section of mouse intestine was imaged using multiphoton microscopy with the RMS1000 Confocal Raman microscope. The RMS1000 can be equipped with an external femtosecond laser and TCSPC lifetime electronics for advanced spectral and time-resolved multiphoton imaging techniques such as 2PEF and SHG which augments its core Raman imaging capability.









