Quenching of Fluorescence with Temperature
Reliable fluorescence standards and stable fluorescent probes for bio-analytics and chemistry are as important as sensitive indicator fluorophores that utilise the outstanding property of fluorescence of being highly sensitive to the fluorophore’s micro-environment. One of the observable fluorescence parameters, the fluorescence intensity, can be affected by two distinctively different quenching processes: dynamic or static quenching.1 The fact that a fluorescent molecule can show either dynamic, or static quenching, or a combination of both, limits the chances for this molecule to be used as a standard, where often insensitivity is required over a large range of parameters such like temperature, pH, polarity, concentration, etc. On the other hand, the property of
quenching, in particular that of dynamic quenching, can be used as a sensitive indicator in biological or chemical experiments and assays.
The xanthene dye Rhodamine, has been extensively used for both fluorescence standard2,3 and probe purposes.4,5 However, there is a variety of different forms of Rhodamines, and these variants can be distinctively different in their response to temperature, polarity and pH.
In this technical note we show the quenching of fluorescence with temperature using the FS5 Spectrofluorometer.
By using the extended capabilities of FS5-TCSPC we also record, for the same set of temperatures, the fluorescence lifetimes. We can show that temperature dependence of Rhodamine B is exclusively caused by dynamic quenching.
FS5 Spectrofluorometer
Reliable fluorescence standards and stable fluorescent probes for bio-analytics and chemistry are as important as sensitive indicator fluorophores that utilise the outstanding property of fluorescence of being highly sensitive to the fluorophore’s micro-environment. One of the observable fluorescence parameters, the fluorescence intensity, can be affected by two distinctively different quenching processes: dynamic or static quenching. The fact that a fluorescent molecule can show either dynamic, or static quenching, or a combination of both, limits the chances for this molecule to be used as a standard, where often insensitivity is required over a large range of parameters such like temperature, pH, polarity, concentration, etc.
On the other hand, the property of quenching, in particular that of dynamic quenching, can be used as a sensitive indicator in biological or chemical experiments and assays. The xanthene dye Rhodamine, has been extensively used for both fluorescence standard and probe purposes. However, there is a variety of different forms of Rhodamines, and these variants can be distinctively different in their response to temperature, polarity and pH.
Methods and Materials for the Quenching of Fluorescence
Spectral temperature quenching experiments were run with the FS5 Spectrofluorometer in standard configuration with a 150 W xenon lamp and a single photon counting photomultiplier (PMT) detector (Hamamatsu, R928P). Time resolved fluorescence measurements were made by Time-Correlated Single Photon Counting (TCSPC) with 478 nm excitation provided by a pulsed picosecond diode laser (EPL-485). For these fluorescence decay measurements an additional 515 nm long-pass filter was used to block residual excitation from being detected.
A thermoelectrically cooled/heated cuvette holder cassette (SC-25) with integrated controller (TC-125) was used to set and monitor the temperature of the sample. The temperature was monitored for 5 min before each spectral and time-resolved measurement to allow the sample to thermally stabilise. The entire measurement sequence from 5oC to 70oC in intervals of 5oC (with stabilisation times and automated measurement stop conditions) is fully computer controlled by means of the spectrometer’s operating software, Fluoracle®. This applies for both spectral and lifetime measurements. Fluoracle® also contains standard reconvolution fit analysis software. Fluorescence lifetimes T were obtained by fitting to a single exponential model equation I(t)=A+Be(−t/T). Solutions of Rhodamine-B (Sigma-Aldrich, 234141) were prepared in water (Sigma-Aldrich, 95283) in 1 cm quartz cuvettes to an OD525nm=0.2 to keep aggregation effects to a minimum.
Results and Discussion
The temperature dependent emission spectra of Rhodamine-B in water are displayed in Figure 1 from 16 different temperatures between 5°C to 80°C. The emission intensity increases as temperature decreases. As the absorbance stays constant over this temperature range (results not shown) we conclude that this is consistent with a change in the fluorescence quantum yield.
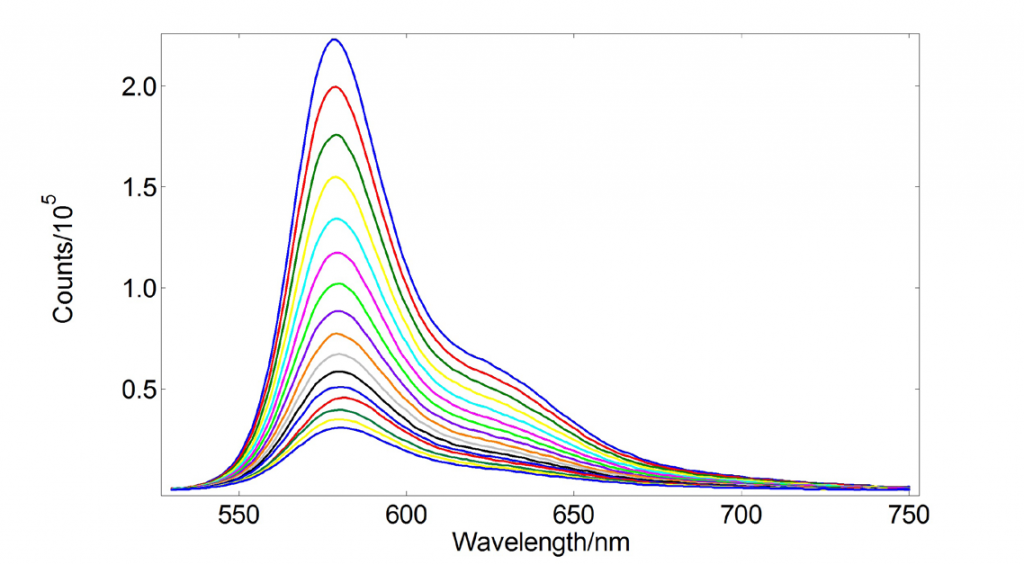
Figure 1: Emission spectra of Rhodamine-B in H2O for temperatures 5°C – 80°C. The parameters of the measurements were λexc=525 nm, Δλexc=Δλem=1 nm, step=1 nm, dwell=1 s, tstab=5 min
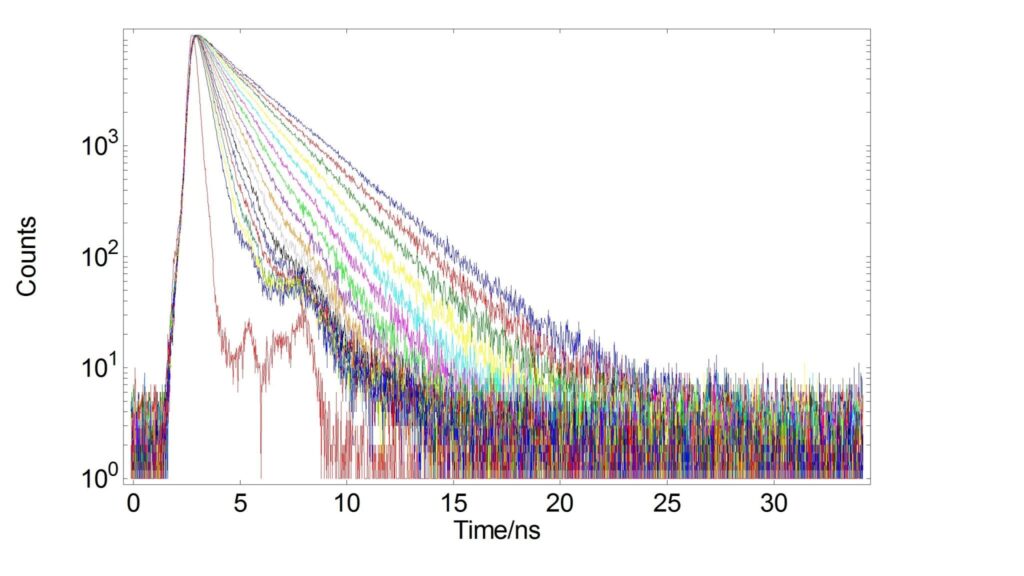
Temperature dependent changes of Rhodamine-B in a different solvent have also been shown in literature2 and explained with mobility changes of the molecule’s diethylamino groups.7 The temperature also affects the radiative lifetime as shown in Figure 2 for the same temperature range.
Figure 2: Radiative decays of Rhodamine-B in H2O from 5°C – 80°C. Measurement parameters were λexc=478 nm, λexc=575 nm, Δλem=5 nm, tcal=0.024 ns, tstab=5 min.
Quenching and temperature dependence of fluorescence in Rhodamine-B has been presented in this technical note. The effect of temperature in the fluorescence and lifetime was readily monitored with the aid of a temperature controlled SC-25 cassette via steady-state and timeresolved techniques. The strong effect of temperature was demonstrated and is associated with dynamic quenching of the fluorescence by temperature related mobility changes of groups within the Rhodamine-B molecule structure. The presented measurements can be further used for estimating the binding constants of new fluorophores.
The integrated fluorescence intensities of the measurements shown in Figure 1 and the fluorescence lifetimes, T, obtained by numerical reconvolution from the measurements shown in Figure 2 are summarised in Figure 3.
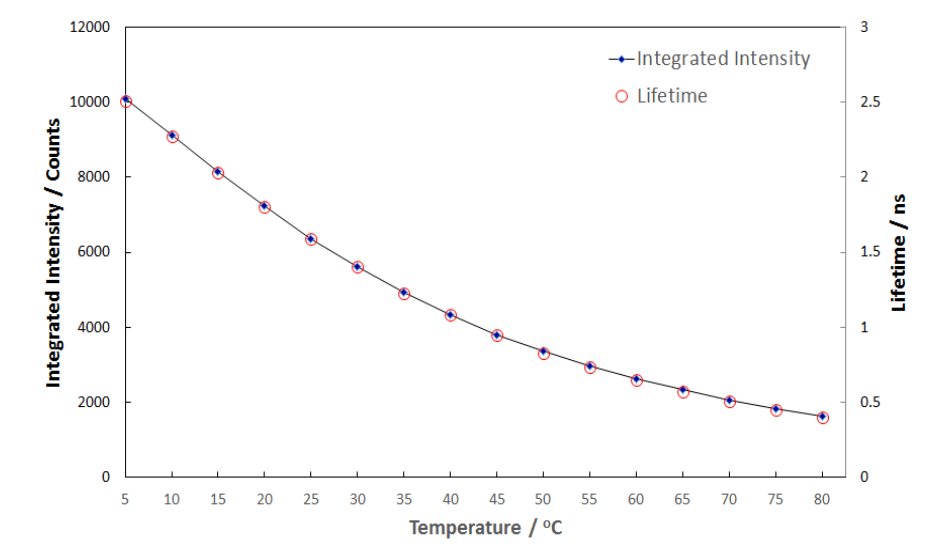
Figure 3: Integrated intensity and lifetime of Rhodamine-B as a function of temperature.
The perfect overlay demonstrates that the change in the fluorescence lifetime is the only cause for the change of the fluorescence intensity. This clearly proves the pure dynamic quenching of the Rhodamine-B fluorescence by temperature increase. At high temperatures, diffusion occurs faster, which leads to greater collisional quenching.1 The results can be further used to perform additional calculations, such as monomer-dimer binding constants.8
Conclusion
Quenching and temperature dependence of fluorescence in Rhodamine-B has been presented in this technical note. The effect of temperature in the fluorescence and lifetime was readily monitored with the aid of a temperature controlled SC-25 cassette via steady-state and time-resolved techniques. The strong effect of temperature was demonstrated and is associated with dynamic quenching of the fluorescence by temperature related mobility changes of groups within the Rhodamine-B molecule structure. The presented measurements can be further used for estimating the binding constants of new fluorophores.
References
- Albrecht, C. Joseph R. Lakowicz: Principles of fluorescence spectroscopy, 3rd Edition. 390, (2008). pp. 278-280
- Karstens, T. & Kobs, K. Rhodamine B and rhodamine 101 as reference substances for fluorescence quantum yield measurements. J. Phys. Chem. 84, 1871–1872 (1980).
- Kubin, R. F. & Fletcher, A. N. Fluorescence quantum yields of some rhodamine dyes. Journal of Luminescence 27, 455–462 (1982).
- Miao, X., Wilson, B. K., Pun, S. H. & Lin, L. Y. Optical manipulation of micron/submicron sized particles and biomolecules through plasmonics. Opt. Express, OE 16, 13517–13525 (2008)
- Zhang, X.-F., Zhang, Y. & Liu, L. Fluorescence lifetimes and quantum yields of ten rhodamine derivatives: Structural effect on emission mechanism in different solvents. Journal of Luminescence 145, 448–453 (2014).
- Selwyn, J. E. & Steinfeld, J. I. Aggregation of equilibriums of xanthene dyes. J. Phys. Chem. 76, 762–774 (1972).
- Lopez Arbeloa, F., Lopez Arbeloa, T., Tapia Estevez, M. J. & Lopez Arbeloa, I. Photophysics of rhodamines: molecular structure and solvent effects. J. Phys. Chem. 95, 2203–2208 (1991).
- Kemnitz, K. & Yoshihara, K. Entropy-driven dimerization of xanthene dyes in nonpolar solution and temperature-dependent fluorescence decay of dimers. J. Phys. Chem. 95, 6095–6104 (1991).
Download the Quenching of Fluorescence Technical Note
Download the complete technical note: Quenching of Fluorescence with Temperature.
FS5 Spectrofluorometer (For measuring Fluorescence Intensity and the Quenching of Fluorescence)
Designed to achieve optimal sensitivity, resolution, and acquisition speed, the FS5 Spectrofluorometer provides the highest measurement specifications in the research and analytical markets. For further information on our FS5 Spectrofluorometer, or our research on fluorescence intensity and the quenching of fluorescence, why not contact one of our sales team at sales@edinst.com.
Stay in Touch
If you have enjoyed learning about the quenching of fluorescence and fluorescence intensity, and want to be the first to see all the latest news, applications, and product information, then sign-up to our infrequent newsletter via the red sign-up button below, and follow us on social media.