ATR-FTIR of Blood Serum Using a Heated ATR Accessory
Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy is a fantastic technique for analysing biofluids. By measuring molecular bond vibrations when interrogated with IR light a biological fingerprint can be obtained from the sample in the form of an IR spectrum. ‘Biofluid biopsies’ are of interest to healthcare researchers and clinicians because they represent a minimally invasive sample collection method. Many biofluids, such as urine, saliva, and blood, can be studied using ATR-FTIR. Spectral differences between biofluid samples allows differentiation between healthy and diseased patients.
Blood is the most widely used biofluid in diagnostic medicine, it is composed of plasma, red blood cells, white blood cells, and platelets. For spectroscopic analysis, plasma, or serum is typically used, this is because both solutions can be frozen for preservation. In whole blood the freezing process would result in cell disruption and the haemoglobin has a large spectral influence. Plasma is an aqueous solution which is separated by centrifuging in an anti-coagulant tube, while serum is created by separating the blood cells and platelets by allowing the blood to clot, Figure 1. Serum is more commonly used as this preparation method is more efficient at removing red blood cells.
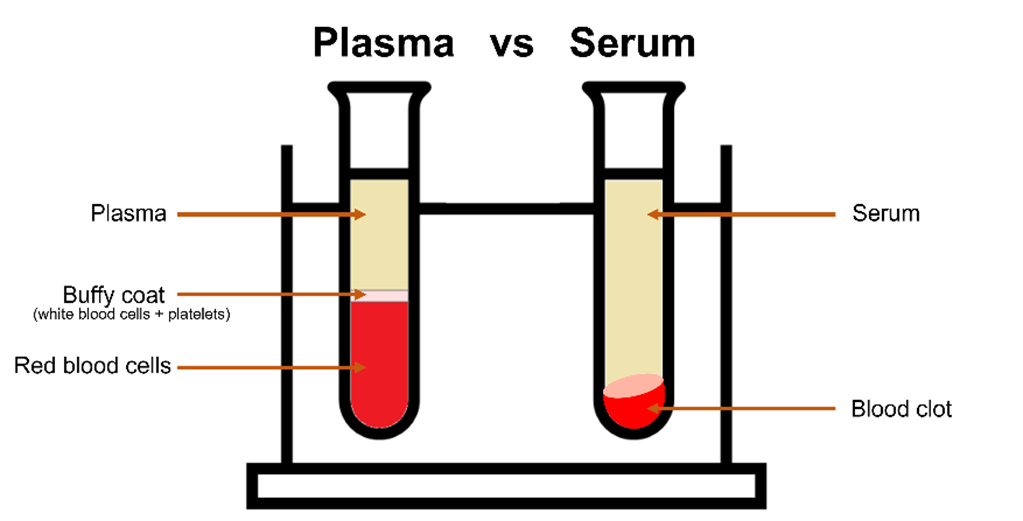
Figure 1: Plasma vs serum.
ATR-FTIR in the mid-IR region (4000 cm-1 – 400 cm‑1) contains the fundamental vibrations of the functional groups in biological samples, such as proteins, lipids, and amino acids. The technique requires a small sample volume in the order of microlitres providing rapid results whilst also being a cost-effective and easy-to-use method for diagnostics. Serum analysis using ATR-FTIR spectroscopy has been demonstrated for a number of disease studies such as cancer, endometriosis, brain disorders, and viral infections.1–6
Impact of Water on Biofluid Measurements
One drawback of ATR-FTIR for biofluid analysis is the influence of water on the IR spectrum, Figure 2. Water produces a strong IR response due to its polarity, and when biofluids are analysed in liquid phase using ATR-FTIR the water spectrum masks the biological information from the sample. Standardly, when measuring biofluids, the user must wait for the sample to dry and reveal the biological spectrum, however, this is time-consuming and slows down the total acquisition time. A faster approach is to use a heated ATR accessory. By heating the sample, the water will evaporate faster, and the total acquisition time is significantly reduced. In this application note, an IR5 FTIR Spectrometer equipped with the heated ATR accessory was used to remove water and rapidly measure the spectrum of serum.
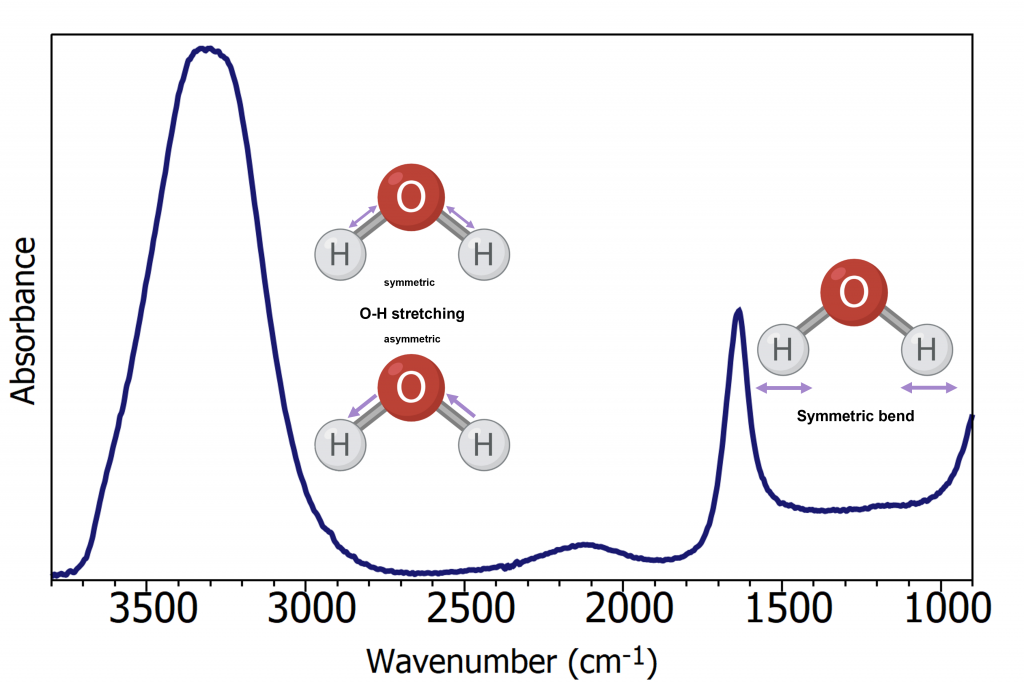
Figure 2: ATR-FTIR spectrum of water.
Materials & Methods
A sample of Human serum albumin was measured in an Edinburgh Instruments IR5 FTIR Spectrometer equipped with a heated ATR accessory. The ATR’s internal reflective element (IRE) was diamond, a commonly used IRE due to its high refractive index and robustness. As a result of their excellent thermal conductivity diamond ATRs are particularly suited to heated experiments.

Figure 3: Edinburgh Instruments IR5 FTIR Spectrometer.
To demonstrate the effect that heating the ATR has on drying times, 3 μL of sample was pipetted onto the ATR without heating and spectra were collected. This process was repeated with the ATR pre-heated and held constant at 50°C. The IR spectra were collected with a resolution of 4 cm-1 averaging 10 spectra with a total acquisition time of 35 seconds for each spectrum.
Results & Discussion
The IR spectra of Human serum albumin acquired after 2 minutes drying on a room temperature (blue) and heated (red) ATR are shown in Figure 4. In the room temperature ATR measurement, the spectrum is dominated by the strong water absorption bands and the biological spectrum is obscured. While for the heated ATR a full biological spectrum without any water interference was obtained. If the blue spectrum was left to dry longer at room temperature it would take over 15 minutes of drying for the biological spectrum to be sufficiently revealed for analysis, greatly reducing measurement throughput.
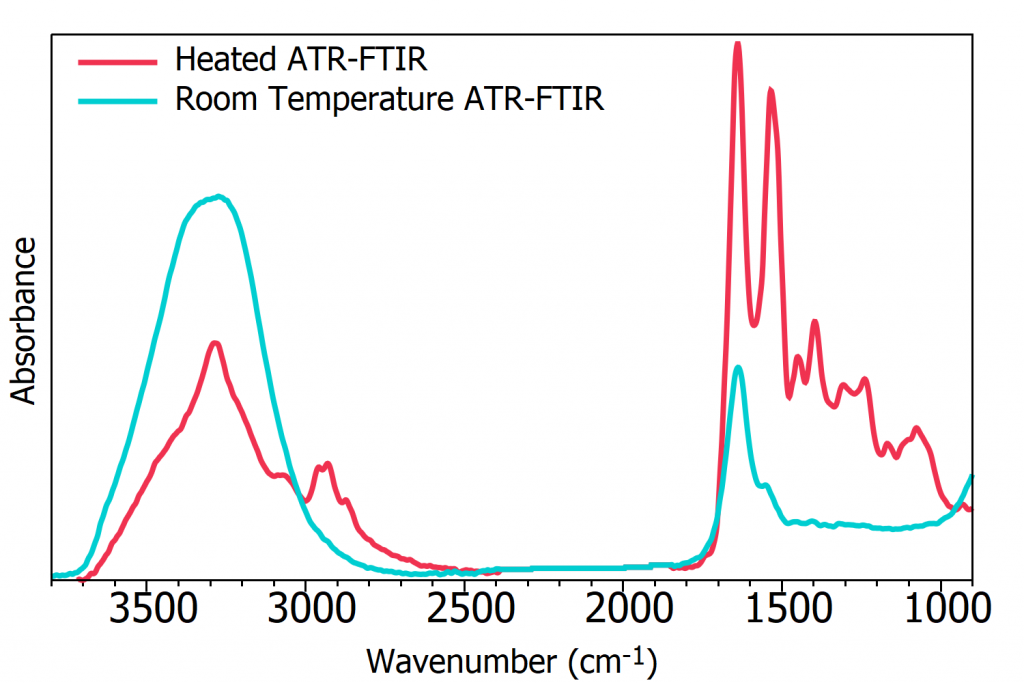
Figure 4: ATR-FTIR spectra after 2 minutes drying time of Human serum albumin at room temperature (blue) and heated to 50ׄ°C (red).
ATR-FTIR reveals significant information on the biological components within a biofluid sample and Figure 5 gives the band assignments of the serum spectrum. IR bio-spectra can be thought of as two regions: the high-wavenumber region from 2600 cm-1 to 3800 cm-1, and the low-wavenumber region from 2000 cm-1 to 500 cm-1 consisting of double bond stretching and the fingerprint region. The low-wavenumber region produces the most discriminatory data, and is the area most used for diagnostics. 7
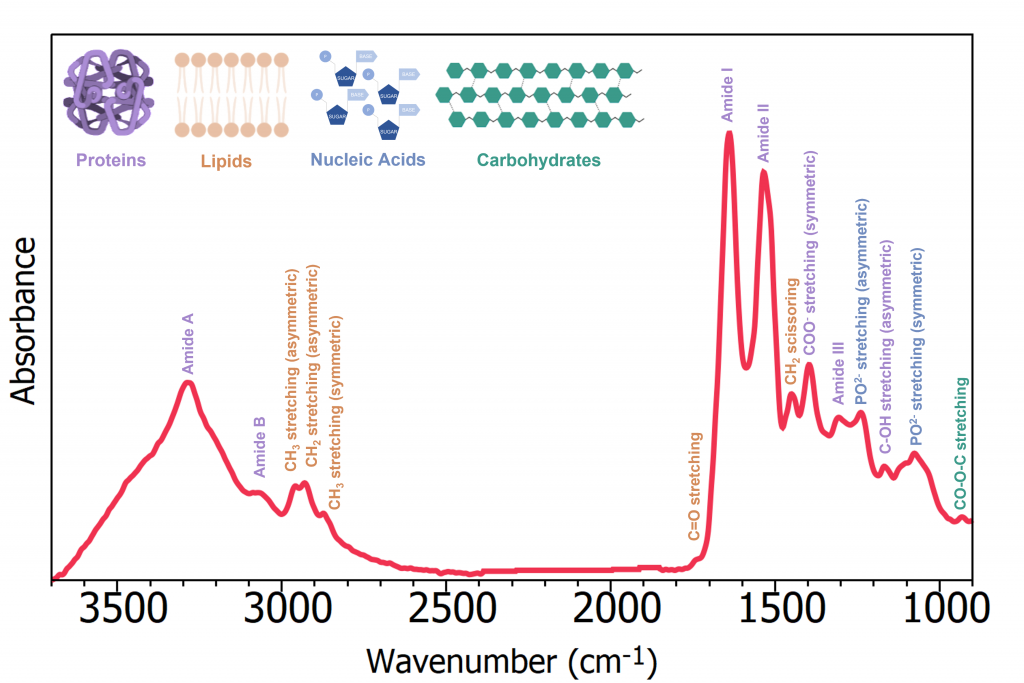
Figure 5: ATR-FTIR spectrum with band assignments of Human serum.
Conclusion
This application note demonstrates the high sensitivity of the IR5 to the analysis of biofluids by providing a biological fingerprint of a serum sample using ATR FTIR Spectroscopy. The problem of parasitic water absorption was overcome by equipping the IR5 with a heated ATR accessory. By using a heated ATR the drying time required before spectral acquisition was greatly reduced without reducing the spectral quality.
References
- T. Soares Martins et al., “Potential of FTIR Spectroscopy Applied to Exosomes for Alzheimer’s Disease Discrimination: A Pilot Study,” J. Alzheimer’s Dis., vol. 74, no. 1, pp. 391–405, 2020
- L. J. Pabico et al., “Diagnostic Efficiency of Serum-Based Infrared Spectroscopy in Detecting Breast Cancer: A Meta-Analysis,” Lab. Med., vol. 54, no. 1, pp. 98–105, 2023
- S. Guo et al., “Fast and Deep Diagnosis Using Blood-Based ATR-FTIR Spectroscopy for Digestive Tract Cancers,” Biomolecules, vol. 12, no. 12, pp. 1–15, 2022
- S. Roy et al., “Spectroscopy goes viral: Diagnosis of hepatitis B and C virus infection from human sera using ATR-FTIR spectroscopy,” Clin. Spectrosc., vol. 1, p. 100001, 2019
- K. Naseer et al., “ATR-FTIR spectroscopy as the future of diagnostics: a systematic review of the approach using bio-fluids,” Appl. Spectrosc. Rev., vol. 56, no. 2, pp. 85–97, 2021
- I. Kokot et al., “ATR-IR Spectroscopy Application to Diagnostic Screening of Advanced Endometriosis,” Oxid. Med. Cell. Longev., vol. 2022, 2022
- A. Rohman et al., “The use of FTIR and Raman spectroscopy in combination with chemometrics for analysis of biomolecules in biomedical fluids: A review,” Biomed. Spectrosc. Imaging, vol. 8, no. 3–4, pp. 55–71, 2019
Keep in Touch
If you have enjoyed this application note, be sure to subscribe to our newsletter and follow us on social media using the links below to keep up to date with our latest product and application news.









