Flexible zinc-air batteries (FZABs) are a potential energy source to power wearable technologies, medical devices, and aerospace components, and there has been significant progress in their development in recent years. They have the advantage of being low-cost, safe, environmentally friendly and having a large theoretical energy density.1
Figure 1 shows the structure of an FZAB and the charging/discharging processes. During discharge, oxygen from the surrounding atmosphere permeates the air cathode and is reduced on the surface of the electrocatalyst (ORR). This is reversed when the battery is charged, and oxygen is released back into the atmosphere (OER).
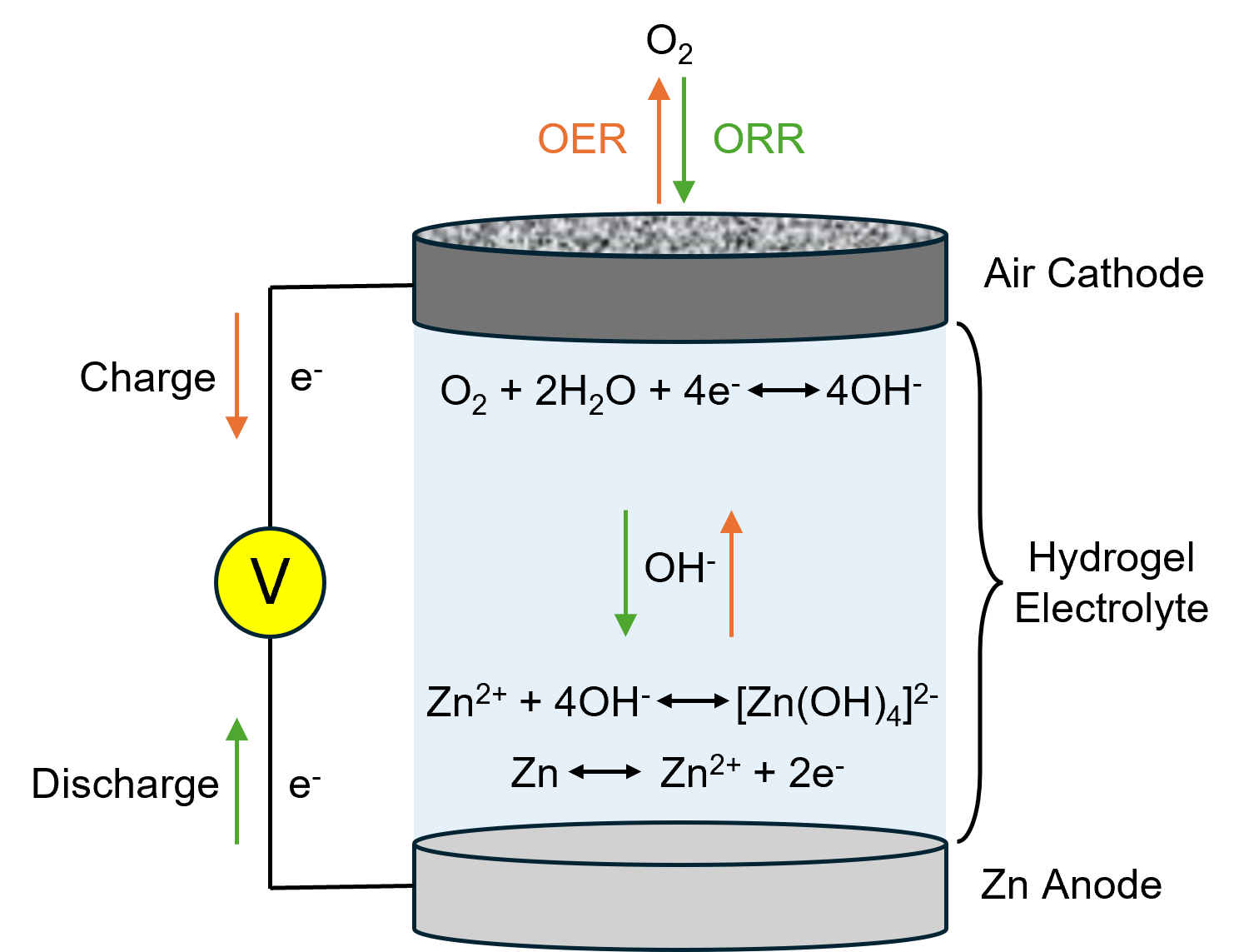
Figure 1. Flexible zinc-air battery structure.
Despite the technology’s numerous advantages, the mechanical properties and conductivity of the FZAB electrolyte will decrease significantly when exposed to low temperatures because of the formation of ice crystals. This reduces the efficacy of using these batteries in technologies that are regularly exposed to subzero temperatures. This issue must be addressed to improve the functionality and stability of flexible electronic devices.
This Research Highlight covers work published in the Journal of Materials Chemistry A by researchers at Guangxi University in Nanning, China.2 They designed a FZAB with a cellulose nanofiber (CNF)-based flexible hydrogel electrolyte containing lithium chloride (LiCl).
They found that the formation of ice crystals at low temperatures was reduced with increasing LiCl concentration. An Edinburgh Instruments RM5 Confocal Raman Microscope was used to confirm the structure of the electrolyte and understand the effect of LiCl content on ice crystal growth inhibition in the hydrogel.
The CNF-based hydrogel electrolyte was designed for a sandwich-structured FZAB comprising a FeCo nanosheet catalyst as the air cathode and a Zn anode. The electrolyte contained CNFs and sodium polyacrylate (PANa) as the crosslinking network and LiCl to inhibit ice crystal growth, Figure 2.
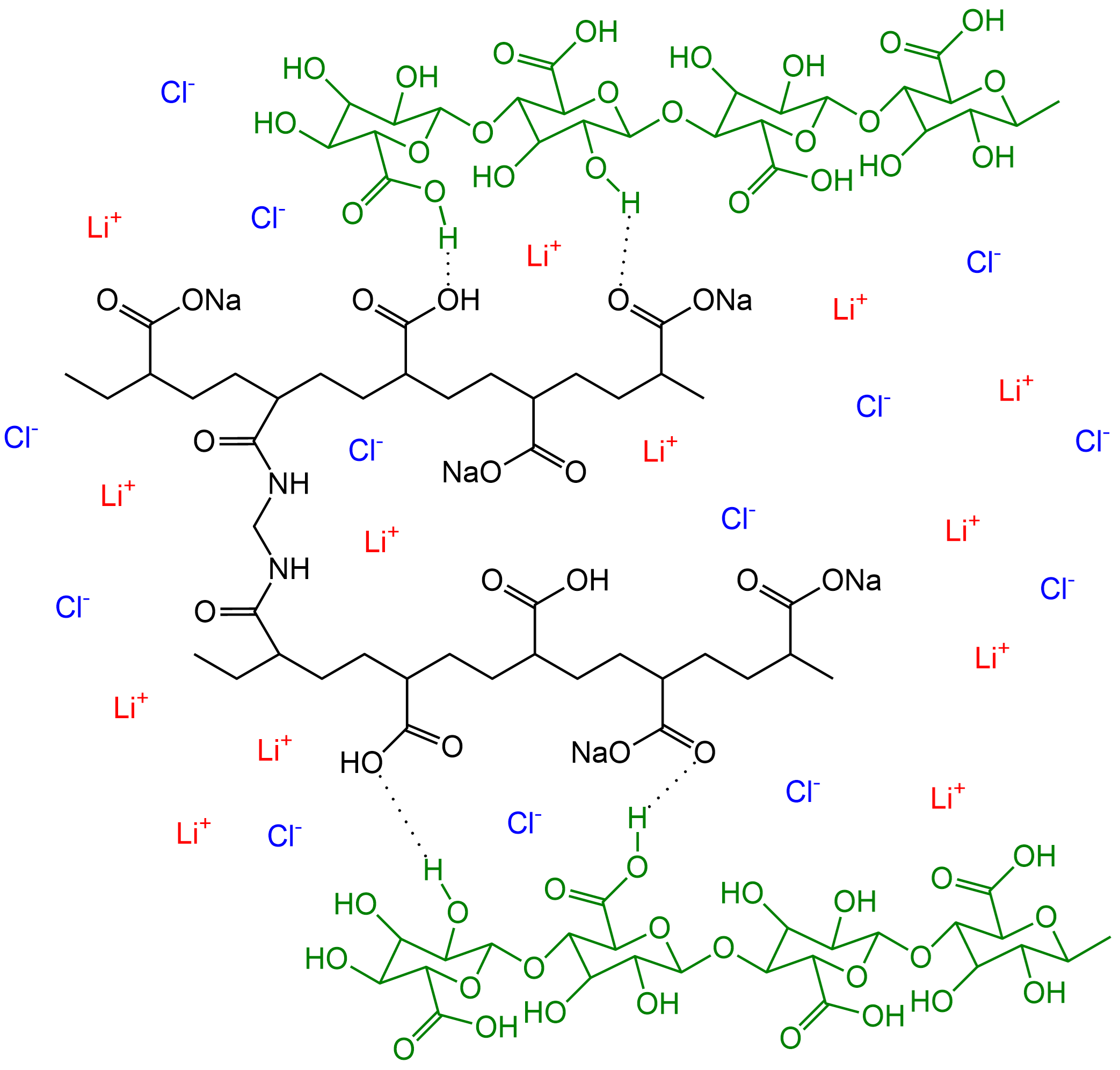
Figure 2. Cellulose nanofiber-based flexible hydrogel structure.
The structure of the hydrogel electrolyte was characterised using an RM5 equipped with a 532 nm laser, Figure 3. The spectral range was set between 400 cm-1 and 4000 cm-1.

Figure 3. Edinburgh Instruments RM5 Confocal Raman Microscope.
The morphology of CNF-based flexible hydrogels (CNF-FH) with differing concentrations of LiCl and a PANa control was characterised using Raman microscopy, Figure 4. The spectral signatures of note in the gels were bands at 1117 cm-1, 1295 cm-1, 1344 cm-1, 1443 cm-1, and 1655 cm-1, which were assigned to C-C skeletal stretching, CH2 twisting, CH bending, CH2 bending, and C=O stretching, respectively. As the concentration of LiCl in the CN-FH was increased, various changes were noted in the Raman spectrum of the material. The intensity of the CH bending mode increased, the CH2 twisting and C=O stretching modes decreased in intensity, and splitting occurred in the CH2 bending mode. These changes were all attributed to Cl– ions becoming embedded in the hydrogel structure and changing the hydrogen bonding.
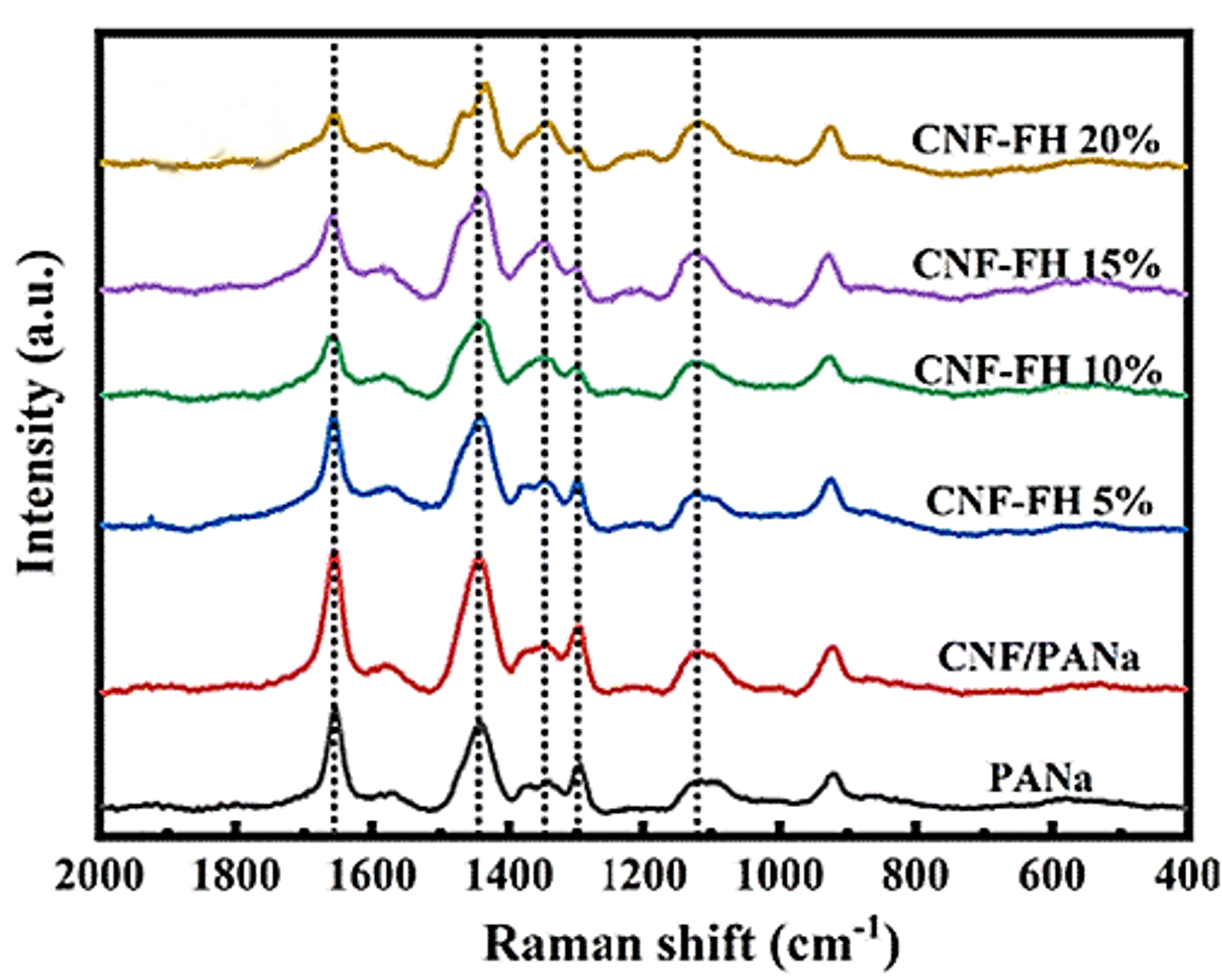
Figure 4. Raman spectra of PANa, CNF/PANa, and CNF-FH structures with varying concentrations of LiCl. The image is reprinted from Xue et al.2, Copyright (2022), with permission from the Royal Society of Chemistry.
To better understand why LiCl reduced ice crystal formation in the FZAB electrolyte at low temperatures, the region corresponding to water vibrational modes in the Raman spectra of the different electrolytes was analysed, Figure 5.
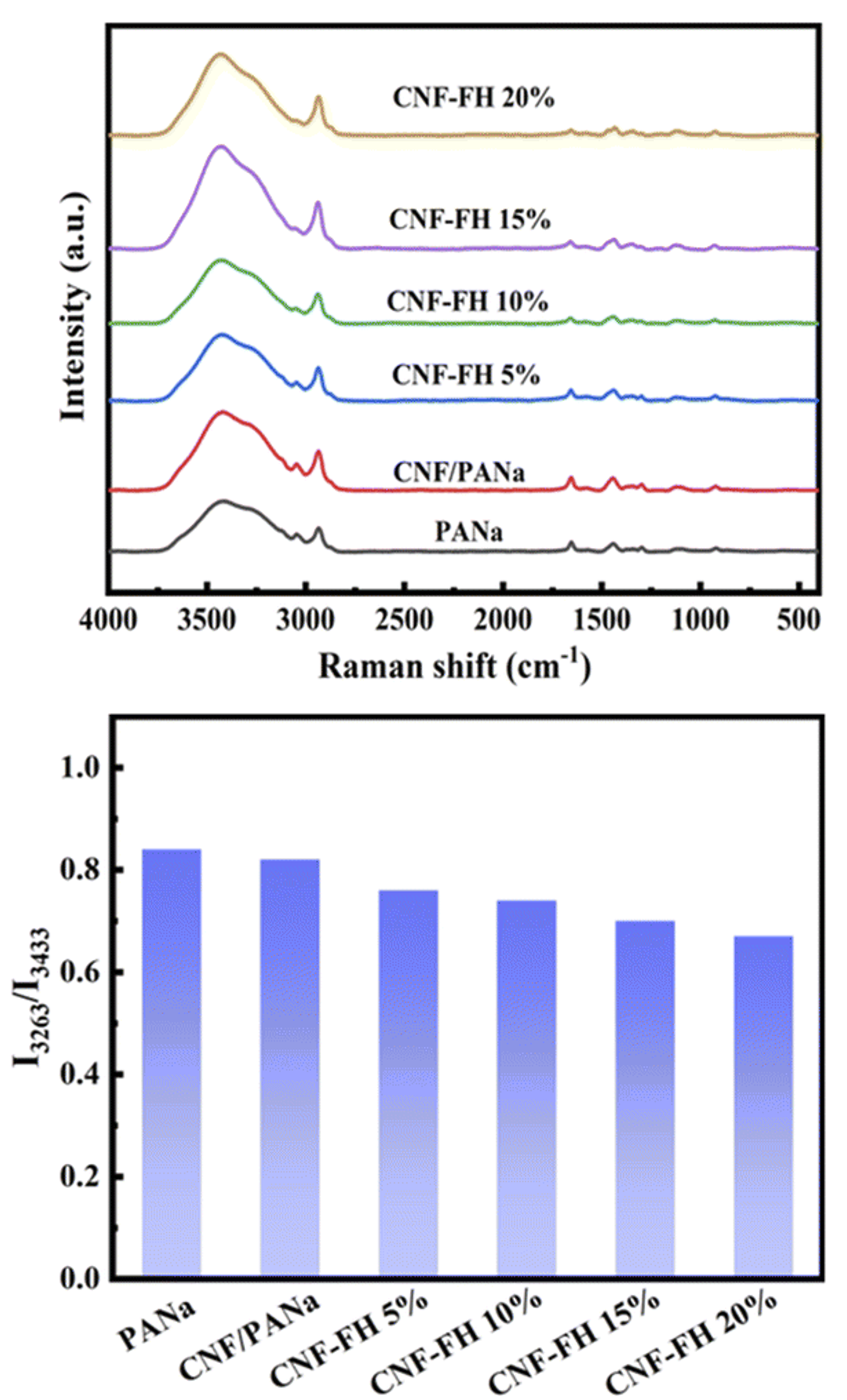
Figure 5. (a) Raman spectra of water in PANa, CNF/PANa, and CNF-FH structures and (b) peak intensity ratios of the symmetric and asymmetric OH stretching bands. The image is reprinted from Xue et al.2, Copyright (2022), with permission from the Royal Society of Chemistry.
It was observed that as the LiCl concentration was increased in the CNF-FH, the intensity ratio between the symmetric OH stretching band at 3263 cm-1 and the asymmetric OH stretching band at 3433 cm-1 decreased. The ratio between these bands is a key marker of the water-ice phase transition, with the symmetric band dominant in ice and the asymmetric band dominant in water. This difference is attributed to the degree of order in the sample, with asymmetric OH stretching being much more common when hydrogen bonds can both form and break in a flexible structure and symmetric OH stretching being common in rigid structures such as ice.3 Therefore, the dampening of the symmetric OH band relative to the asymmetric suggests that LiCl is weakening the ability of the water molecules to form the hydrogen bonds required for crystallisation. These results confirmed the ability of the LiCl to inhibit ice formation at low temperatures.
This Research Highlight showed how Raman microscopy can be applied to improve electrolyte design in FZABs. In this work, the technique was used to characterise a cellulose nanofiber-based flexible hydrogel electrolyte and better understand the mechanisms through which LiCl inhibited the formation of ice crystals in the batteries at low temperatures. This promising study could increase the efficacy of using flexible batteries in technologies designed for aerospace components and automotive or wearable electronics regularly exposed to subzero temperatures.
The results in this Research Highlight were published in the Journal of Materials Chemistry A. The full article can be found here: https://pubs.rsc.org/en/content/articlelanding/2022/ta/d2ta06782j.