Scintillators are materials that convert high-energy, ionising radiation into lower-energy, UV or visible light. This is important for the detection of X-rays in medical imaging, particle physics research, and security scanners. Whilst conventional scintillators such as cerium-doped lutetium yttrium orthosilicate (LYSO:Ce) and bismuth germanate (BGO) have many advantages, they suffer from poor chemical stability in the presence of moisture, acids, bases, and polar solvents.
Novel materials such as copper (I) clusters are seen as more robust alternatives with strong X-ray absorption, tuneable emission, and stability against moisture. However, these cluster-based materials often exhibit losses in efficiency due to radioluminescence (RL) quenching.
This Research Highlight presents work published in Advanced Optical Materials by Chang Gu, Tao Wang, Xian Qin, Xiaogang Li.1 In this work, the authors introduced halogen-containing ligands to copper (I) clusters in order to improve lattice symmetry and rigidity, with the aim of enhancing RL quantum yield (Figure 1).
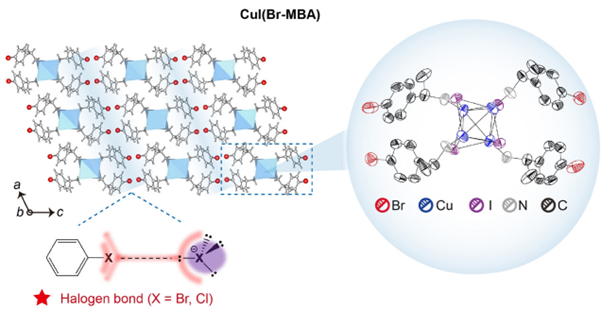
Figure 1: Crystalline lattice structure and packing of halogenated cluster, CuI(Br-MBA). Bottom inset highlights halogen bonding between the organic ligand and the CuI moiety. Right inset shows a magnified view of the crystal structure, and the coordination environment of the clusters. Reprinted with permission from Gu et al.1
A halogen-bonded Cu(I) cluster scintillator with the formula Br-MBA)4Cu4I4, or CuI(Br-MBA), was synthesised (MBA = S-methylbenzylamine). The optical properties of this compound were compared to a cluster with halogen-free ligands, CuI(MBA).
The photoluminescence (PL) spectra and quantum yield (PLQY) of the clusters were determined using an Edinburgh Instruments FLS1000 Photoluminescence Spectrometer equipped with an integrating sphere. RL spectra were collected on an EI FS5 Spectrofluorometer under excitation from an X-ray source. Temperature-dependent RL measurements were performed on an Edinburgh Instruments FLS980 spectrofluorometer coupled to a heating and cooling stage.
PL measurements
CuI(MBA) possessed excitation and emission maxima at 352 nm and 596 nm, respectively (Figure 2a). CuI(Br-MBA) exhibited excitation and emission maxima at 351 nm and 575 nm, respectively (Figure 2b). As the ligands were not contributing to the emission, luminescence was assigned to Cu4I4 cubanes in the lattice. Introduction of halogens into the structure caused a marked enhancement in PLQY, increasing from 35.7% in CuI(MBA) to 89.9% in CuI(Br-MBA) (Figure 2c). It was hypothesised that the presence of halogens in the crystal structure increased lattice symmetry, reducing vibronic coupling and trap formation, and therefore minimising non-radiative decay (Figure 2d).
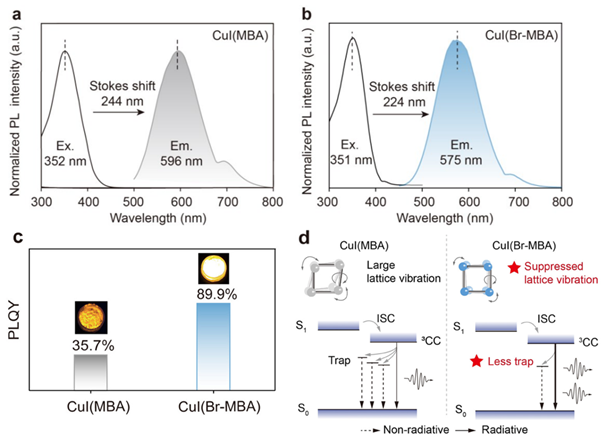
Figure 2: (a) Normalised excitation and emission spectra for CuI(MBA). (b) Normalised excitation and emission spectra for CuI(Br-MBA). (c) Absolute PLQY of CuI(MBA) and CuI(Br-MBA). (d) Proposed mechanism for the improved quantum efficiency of the halogenated cluster. The improved symmetry of CuI(Br-MBA) results in reduced lattice vibration, fewer traps, and therefore suppression of non-radiative pathways. Reprinted with permission from Gu et al.1
RL Measurements
Under excitation from an X-ray source, both CuI(MBA) and CuI(Br-MBA) exhibited RL spectra analogous to the PL spectra, confirming emission was proceeding via Cu4I4 cubanes (Figure 3a). Next, the performance of the Cu-clusters was compared to established scintillators BGO and LYSO:Ce. The light yield of CuI(Br-MBA) (28539 photons MeV-1) outperformed CuI(MBA) (2128 photons MeV-1) and BGO (8500 photons MeV-1) and exhibited 82% of LYSO:Ce (34559 photons MeV-1 (Figure 3b). As well as showing comparable or improved efficiency with respect to LYSO:Ce and BGO, CuI(Br-MBA) exhibited a 13-fold increase in RL efficiency with respect to CuI(MBA).
The detection limit describes the lowest dose of X-rays that a scintillator can accurately detect. To measure this, the RL intensity of the clusters was measured at decreasing X-ray dose. CuI(Br-MBA) and CuI(MBA) exhibited detection limits of 28.8 nGyair s−1 and 73.6 nGyair s−1 respectively, further showcasing the improved properties of the halogenated clusters (Figure 3c). CuI(Br-MBA) also showed high stability to irradiation, reducing in intensity by only 2.1 % over 1800 s of cyclic X-ray exposure (Figure 3d).
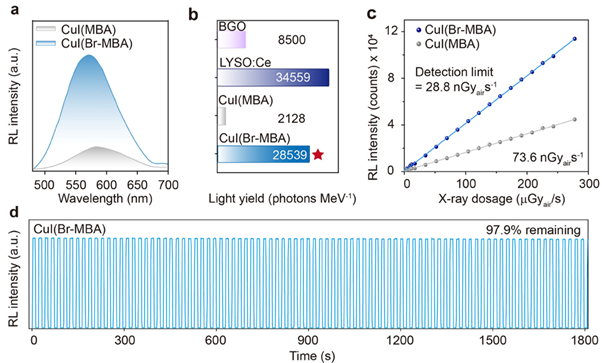
Figure 3: (a) RL emission spectra of CuI(MBA) and CuI(Br-MBA). (b) RL light yield of BGO, LYSO:Ce, CuI(MBA) and CuI(Br-MBA). (c) RL limit of detection of CuI(MBA) and CuI(Br-MBA). (d) Stability in RL intensity of and CuI(Br-MBA) over 30 mins of cyclic X-ray exposure. Reprinted with permission from Gu et al.1
The temperature dependence of the Cu clusters on RL emission was determined. Samples of the two clusters were measured at temperatures from 80 to 380 K. Upon increasing temperature, both clusters exhibited a blue shift in emission wavelength and negative thermal quenching: a phenomenon where emission intensity increases with temperature (Figure 4a). This was attributed to exciton release from traps due to thermal stimulation. These traps are formed by lattice distortion from exposure to high-energy radiation. After a certain temperature was reached (200 K for CuI(Br-MBA) and 300 K for CuI(MBA)), a decrease in emission intensity was observed. The negative thermal quenching effect was less pronounced in CuI(Br-MBA), suggesting reduced defect trapping sites in the halogen-stabilised lattice.
The thermal stability of CuI(Br-MBA) was also tested by measuring RL intensity of the cluster over 30 hours of heating at 100 °C. Over the course of the experiment, the cluster retained 83% of its initial intensity, showing high stability in harsh conditions (Figure 4b).
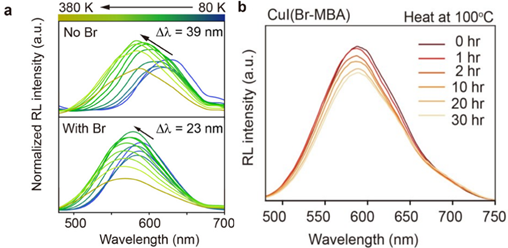
Figure 4: (a) Variable temperature RL of CuI(MBA) (top) and Cu(Br-MBA) (bottom). (b) RL stability over 30 h over of heating at 100 °C. Reprinted with permission from Gu et al.1
Stability Studies
Finally, the stability of the clusters to acids, bases, and polar solvents, was assessed. To do this, samples were immersed in each liquid, and the RL intensity was measured over several days. The change in intensity was normalised to the initial value. In acidic solution (pH 5.5), CuI(MBA) exhibited a huge loss of intensity after 10 days, only retaining 17% of the initial intensity. However, CuI(Br-MBA) was highly stable, retaining 91% of the initial intensity after 10 days (Figure 5a). In a basic solution (pH 8.0) the effects were even more pronounced, with CuI(MBA) and CuI(Br-MBA) retaining 8.6% and 99.8% of initial intensity over 10 days (Figure 5b). In ethanol, CuI(MBA) reached complete RL quenching after 4 days, whereas CuI(Br-MBA) was unchanged after 16 days (Figure 5c). In water, CuI(MBA) and CuI(Br-MBA) were reduced to 33% and 87% of their initial RL intensity, respectively (Figure 5d).
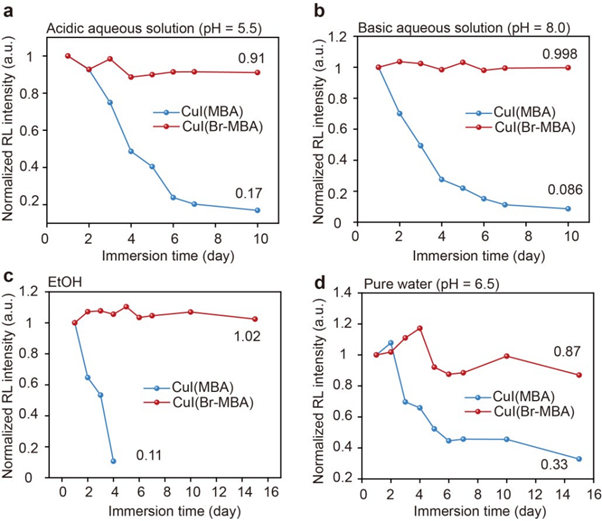
Figure 5: Normalised RL intensity of CuI(MBA) and CuI(Br-MBA) in (a) acidic aqueous solution, (b) basic aqueous solution, (c) ethanol (d) pure water. Reprinted with permission from Gu et al.1
In this work, EI’s FLS series and FS5 spectrometers were used for the characterisation of Cu(I) clusters for their application as X-ray scintillators. These allowed collection of PL spectra, PLQY, as well as RL spectra and temperature-dependent RL. These results showed how introduction of halogens into the lattice of Cu(I) clusters improved their optical properties in several regards. This included an increase in PLQY and RL light yield, with comparable or improved performance with respect to established scintillators BGO and LYSO:Ce. The halogenated cluster, CuI(Br-MBA), also showed a low limit of detection of 28.8 nGyair s−1, high stability to ionising radiation, high temperatures, as well as chemical stability in polar solvents, and acidic and basic conditions.
The full article was published in Advanced Optical Materials.
1 C. Gu, T. Wang, X. Qin, and X. Liu, Adv. Opt. Mater., DOI:10.1002/adom.202501022.