Over the Covid-19 pandemic, hand sanitiser became an essential tool in our improved hygiene practices. As the world starts to open up again, here at Edinburgh Instruments we are excited to be back attending conferences. The sales and applications team were keen to get on the road again and discuss our work with current and potential customers, but we are keen to help everyone stay safe. That’s why we have updated our booth to include giveaways of hand sanitiser. The applications team recently analysed our sanitiser using an RM5 Raman Microscope with cuvette holder attachment to make sure they were effective for hand sanitising.
Figure 1: Cuvette holder (left) used with an RM5 Raman Microscope (right)
Whilst hand sanitiser has been around for years, the pandemic massively increased its use and production. Sales jumped by a massive 600% in 2020. This rise left businesses with huge profit potential if they could manufacture hand sanitiser. Unfortunately, due to the demand, some companies started to make hand sanitiser cheaply, and therefore it was ineffective against COVID-19.
Alcohol is the active ingredient in hand sanitiser, and a concentration of 60% or higher is required to be effective.1 Simply, the higher the percentage of ethanol, the higher the efficacy. Lower concentrations of alcohol may have some effect of bacteria and viruses, such as slowing their growth, but <60% will likely not kill the bugs on your hands. Hand sanitiser works as the alcohol attacks pathogens by damaging their key cell structures, in the case of COVID-19 the alcohol breaks down the envelope of the virus rendering it impossible to infect.
Figure 2: Effect of ethanol on viral cell envelopes
The usual alcohol in hand sanitisers is either ethanol or isopropanol, or a mixture of the two is also common. Many bottles of sanitiser will state a percent alcohol concentration, but during the pandemic some products failed to meet the minimum percentage stated on their own branding. A Which? study in December of 2020 found a product sold on eBay claiming to have a 75% alcohol content actually only contained 10% alcohol.2
Raman spectroscopy is an extremely sensitive technique which uses scattered light to measure the vibrational energy modes of a sample. It’s sensitive to concentration changes and can be used for quantitative analysis. One such type of analysis used by analytical chemists is calibration (or standard) curves. Calibration curves are used to determine the concentration of unknown samples by comparing them to a series of standards of known concentrations.
Ethanol provides a strong Raman response, Figure 3(a), and the band at approx. 880 cm-1 increases as the concentration increases. To create the calibration curve spectra were taken under the same conditions for ethanol with varying volume percentages: 20%, 40%, 60%, 80%, and 100%. The area of the 880 cm-1 peak behaves linearly with increasing ethanol percent and therefore a straight line can be plotted, Figure 3(b). The calibration curve provides the equation of the line which allows an unknown sample to be measured and plotted on the calibration curve
Figure 3: (a) Ethanol Raman spectrum (b) Ethanol calibration curve. Measurement conditions: 785 nm, 300 gr/mm, 0.5 second exposure time, 2 mm pinhole, 100 µm slit.
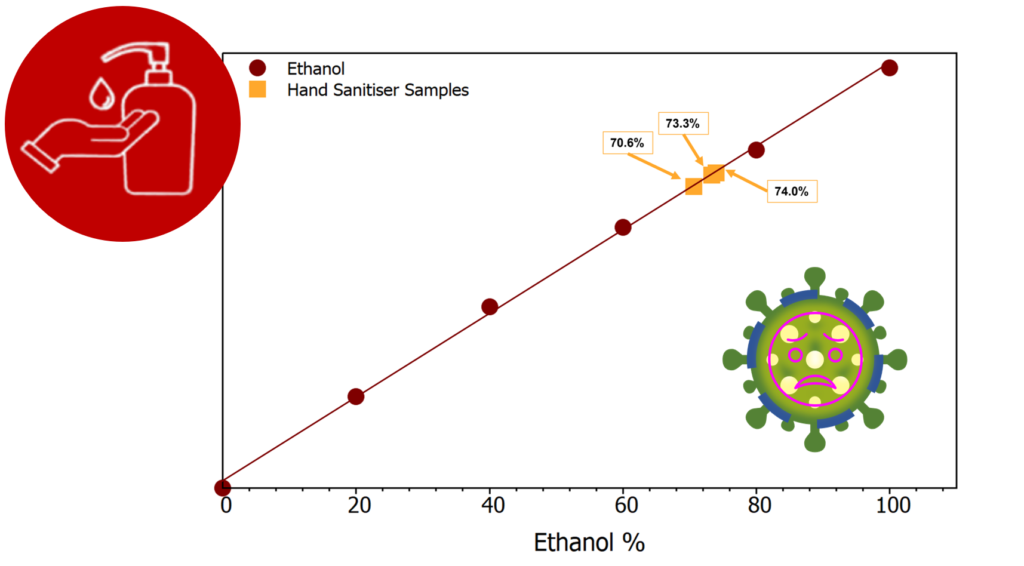
Three bottles of the hand sanitiser were tested to see the variation between bottles from the same batch. The label stated the alcohol content to be at least 68% ethanol, to check this claim a small volume from each bottle was poured into a cuvette. Spectra were acquired for each sample and the area of the 884 cm-1 peak recorded, this value is now the “y” value for the equation of the line and can be used to determine the “x” value which is the ethanol %. Figure 4 shows the 3 samples marked onto the calibration curve (orange).
Figure 4: Calibration curve including 3 hand sanitiser samples
The three bottles of hand sanitiser were proven to have ethanol content >68%, varying from 70.6%-74.0%. This means the hand sanitiser is effective at killing microbes present on your hands, and therefore helps provide a layer of protection against any germs around. Thanks to Raman spectroscopy we now know the hand sanitisers offered are of high quality, giving protection against COVID-19. Make sure to stop by our booth at future events to pick up a bottle and discuss our full range of bespoke instruments that can be tailored to your needs.
If you want to learn more about how the RM5 Raman Microscope can be used for quantitative analysis read the full Application Note or contact our sales team to discuss how the RM5 can be used within your own Raman research.
No results found.

