Photodynamic therapy is a treatment for cancer whereby tumour cells can be destroyed remotely using light. One method to achieve this is by administration of a photosentisiser that induces apoptosis of target cells. Under photoexcitation, the photosensitiser is promoted to a triplet excited state, which reacts with molecular triplet oxygen in the target cells to produce cytotoxic singlet oxygen and reactive oxygen species (ROS).
Ligand-stabilised octahedral molybdenum nanoclusters are seen as attractive photosensitisers due to their high singlet oxygen quantum yield, tunable optical properties, and stability1. This Research Highlight presents a study by Přibyl et al. from the University of Chemistry and Technology Prague and the Czech Academy of Sciences; published in Journal of Materials Chemistry B2. This work reports three molybdenum iodide nanoclusters stabilised by triazolate apical ligands bearing dibenzo[a,e]cyclooctyne (DBCO) moieties with (Figure 1).
![Molecular structure of nanoclusters 1-3 with the formula [Mo6Li8La6]n , and their corresponding apical ligand. Reprinted with permission from Přibyl et al.1](https://edin.becdn.net/wp-content/uploads/2025/07/Molecular-structures-of-nanoclusters-1-3.gif)
Figure 1: Molecular structure of nanoclusters 1-3 with the formula [Mo6Li8La6]n , and their corresponding apical ligand. Reprinted with permission from Přibyl et al.1
An Edinburgh Instruments FLS1000 Photoluminescence (PL) Spectrometer with double excitation and emission monochromators was used to measure singlet oxygen generation by the nanoclusters, their phosphorescence emission, and their lifetimes. Additionally, the FLS1000 in conjunction with the XS1 Radioluminescence (RL) Chamber was used to measure RL emission of the nanoclusters under X-ray excitation. For medical applications this is important as X-rays are not attenuated by soft tissue like visible light is, and thus could be used to induce PDT deep within the body.
Spectral measurements were performed under excitation from a Xe lamp, and time-resolved measurements were performed under excitation from an Edinburgh Instruments VPLED-405 (405 nm) picosecond pulsed source. ROS generation was measured by dosing compounds 1-3 into a solution of ROS sensor, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), and monitoring emission at 525 nm under 488 nm excitation. Spectral RL measurements were performed with an FLS1000 connected to the XS1 RL Sample chamber, with excitation from a X-ray source (60 KeV, 200 mA).
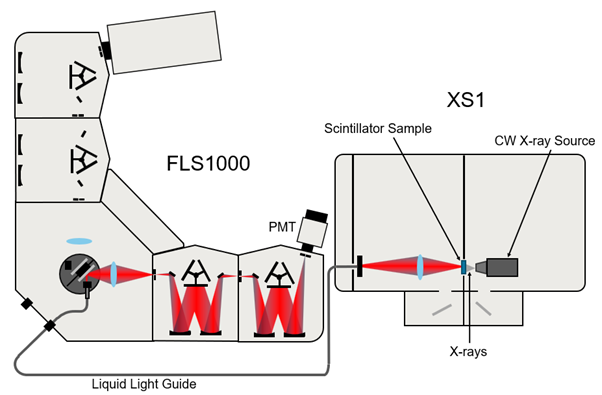
Figure 2: Schematic of the FLS1000 with double excitation and emission monochromators coupled to the XS1 RL Chamber.
Photoluminescence Measurements
The PL of the nanoclusters dissolved in argon-saturated phosphate-buffered saline (PBS) was measured under Xe lamp excitation at 400 nm. Samples 1-3 all emitted in the red region, centred around 690 nm (Figure 3a). Time-resolved measurements of the samples in argon saturated PBS was measured via Multi Channel Scaling, revealing long lifetimes of 79, 78, and 105 μs for 1, 2 and 3, respectively. In contrast, when samples were measured in air-saturated PBS, the lifetimes were reduced to 19, 43, and 22 µs for 1, 2, and 3, respectively (Figure 3b). The relatively long lifetimes and their reduction in contact with triplet quencher, oxygen, gave a strong indication that the luminescence was proceeding via a triplet excited state, which is essential for the PDT application.
Detection of singlet oxygen was performed by observation of a phosphorescence band at 1270 nm. All three samples produced this signal; however, based on the relative signal-to-noise ratio of the peaks, photosensitiser 2 produced the highest levels of singlet oxygen (Figure 3c). This was further confirmed by the use of sensor DCFH-DA, which is oxidised to green fluorescent 2’, 7’-dichlorofluorescein in the presence of ROS. Integration of its green emission peak provided quantification of ROS generation.
For a range of concentrations of the photosensitizers, it was found the greatest and lowest levels of ROS were produced by 2 and 3 respectively (Figure 3d). This trend was also reflected in the degree of lifetime quenching observed previously. The observed variation in reaction between the nanoclusters with DCFH-DA was attributed to differences in the surface charge of their ligands.
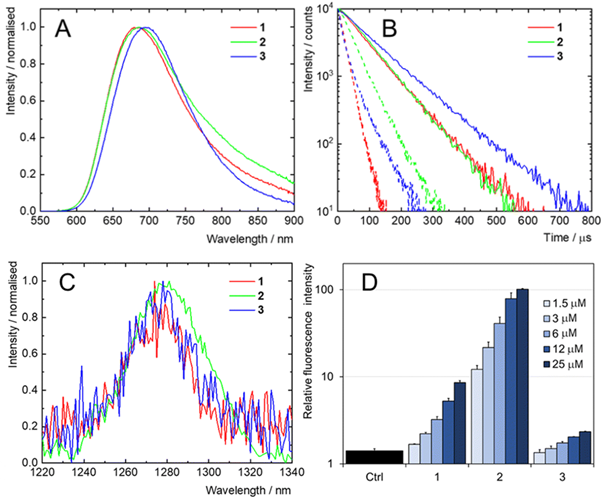
Figure 3: (a) Normalised PL emission of 1-3 in solutions of argon-saturated PBS. (b) PL lifetime of 1-3 in argon saturated (solid lines) and air saturated (dashed lines) PBS under pulsed excitation (405 nm). (c) Normalised PL emission of singlet oxygen produced by 1-3 under photoexcitation. (d) PL emission at 525 nm (Ex = 488 nm) of mixtures of DCFH-DA with increasing concentrations of 1-3 in H2O. Mixtures were irradiated at 460 nm for 5 mins. Control (Ctrl) is DCFH-DA alone. Reprinted with permission from Přibyl et al.2
Radioluminescence Measurements
RL emission from molybdenum nanoclusters in the solid state was measured under X-ray excitation in an aerobic environment. RL emission of the nanoclusters was similar to phosphorescence band (Ex = 400 nm) with the exception of 2, where a small red-shift was observed under X-ray excitation (Figure 4).
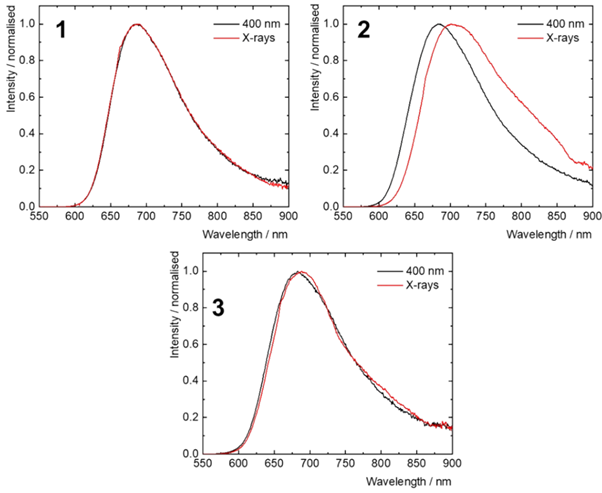
Figure 4: Overlaid normalised PL emission (Ex = 400 nm), and RL emission (Ex = 60 KeV ) of nanoclusters 1-3. Reprinted with permission from Přibyl et al.2
In this Research Highlight, it was shown that the Edinburgh Instruments FLS1000 spectrometer equipped with the XS1 Radioluminescence Chamber can be used for photophysical characterisation of molybdenum nanocluster PDT agents. Spectral and time-resolved PL were used to confirm singlet oxygen generation and determine the phosphorescence lifetime of the nanoclusters. A DCFH-CA sensor was used to quantify ROS generation, showing that nanocluster 2 was the best performing. Lastly, spectral RL measurements showed that the nanoclusters exhibited scintillation, showing similar emission profiles under X-ray and visible excitation.
You can view the full article that was published in Journal of Materials Chemistry B.
1 H. C. D. Medeiros, C. Yang, C. K. Herrera, D. Broadwater, E. Ensink, M. Bates, R. R. Lunt and S. Y. Lunt, Chemistry – A European Journal, DOI:10.1002/chem.202202881.
2 T. Přibyl, K. Škoch, D. Bavol, I. Křížová, J. Zelenka, T. Ruml, K. Lang and K. Kirakci, J Mater Chem B, 2025, 13, 6256–6264.