Additive manufacturing or 3D printing has emerged as a revolutionary approach in various therapeutic applications. In particular for bone tissue engineering, 3D printing offers unprecedented control over the fabrication of scaffolds that can mimic the complex architecture of natural bone and the creation of personalised implants.
Synthetic polymers, such as thermoplastic polyurethane (TPU), have demonstrated great potential in 3D-printed bone tissue engineering. A key advantage of 3D printing bone scaffolds is the ability to embed therapeutic agents into the base material.1 Dipyridamole (DIP), an antiplatelet drug known to enhance bone regeneration, shows significant potential when integrated into 3D-printed bone implants, offering improved bone regeneration with minimal side effects.
Accurate characterisation of drug-infused implants is crucial to confirm uniform drug distribution and ensure effective release when integrated into bone. Raman microscopy is a powerful, non-destructive technique for discriminating drugs in polymer matrices and imaging their distribution (Figure 1).
This Research Highlight presents collaborative work from Queens University Belfast, University of Girona, Ulster University, Universidad de Santander, and Universidad de Sevilla. The study demonstrates successful doping of polymer implants with two concentrations of DIP and detailed characterisation using an RM5 Confocal Raman Microscope. Published in the Journal of Drug Delivery and Translational Research, this work highlights the potential role of advanced spectroscopic techniques in developing next-generation drug-infused 3D-printed bone implants.2
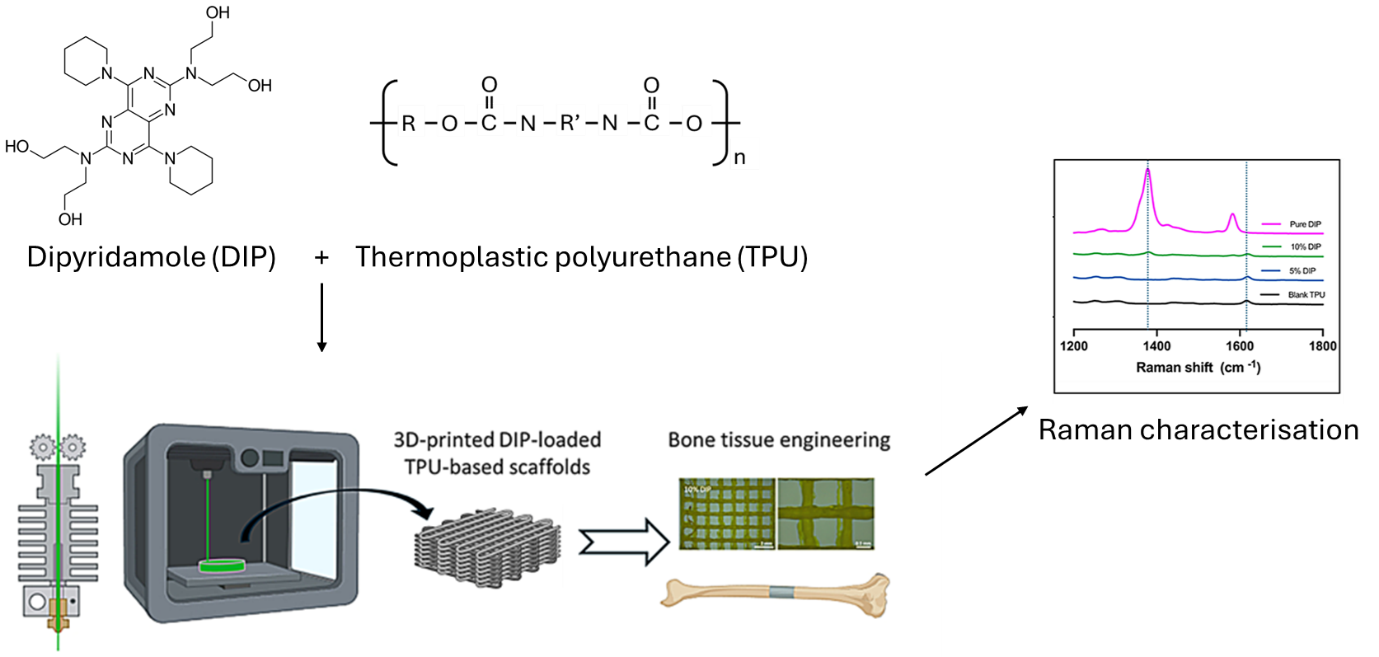
Figure 1. Schematic diagram illustrating the methodology for 3D-printed drug-infused bone and Raman characterisation, adapted from Wang et al.2
Samples of TPU and TPU/DIP (5% and 10%) were prepared following the research group’s previous methodology.1 The final composite material was then designed using computer-aided design (CAD) and an Ultimaker 3 3D printer to produce discs, meshes and rectangular blocks.
Measurements were collected with an RM5 (Figure 2) equipped with a 785 nm laser and 10x 0.25 NA objective lens. Spectra were acquired using a 300 µm pinhole, 70 µm slit and 100% laser power, with a 2.5 second exposure time and three accumulations. Background subtraction was performed using Ramacle® software. Raman images were collected using a 15 x 15 point grid. Contrast was obtained by mapping the peak intensity between 1350-1400 cm-1, attributable to DIP, from each pixel.

Figure 2. RM5 Confocal Raman Microscope.
To ascertain the feasibility of tracking the drug in the polymer scaffold, Raman characterisation was performed on TPU, TPU with 5% DIP, TPU with 10% DIP, and pure DIP (Figure 3). This technique is particularly well-suited for discriminating between the drug and the polymer matrix due to its high spectral resolution, allowing specific chemical bonds to be identified.
In the pure TPU spectra (black), a characteristic peak was observed at 1615 cm−1, attributed to the C=O stretching from the polymer. The pure DIP sample (pink), conversely, displayed an intense peak between 1350−1400 cm−1, which corresponded to C-N stretching.
Both drug-infused samples (TPU/5% DIP and TPU/10% DIP) exhibited the C=O TPU Raman signature. Crucially, the appearance of the C-N peak confirmed the presence of DIP within these samples, as this signature was absent in the pure TPU. Since Raman intensity is proportional to concentration, the intensity of the 1350−1400 cm−1 peak increased with higher DIP loading.
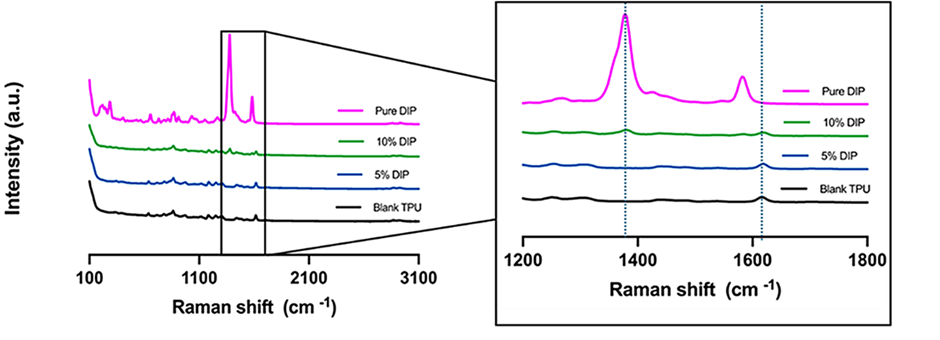
Figure 3. Raman spectra of pure DIP (pink), pure TPU (black) and 3D-printed samples containing TPU/5% DIP (blue) & TPU/10% DIP (green), respectively.
To understand the spatial distribution of DIP, the TPU/10% DIP sample was chosen for Raman mapping. The ability to track individual vibrational modes, and the high spatial resolution offered by coupling Raman with a confocal microscope, means that the distribution of DIP within TPU could be imaged.
The distinct C-N stretching at 1350−1400 cm-1, unique to DIP was used to generate the Raman map. The inherent linearity and concentration-dependence of Raman spectroscopy mean that as DIP loading increases, the intensity of this peak is expected to rise proportionally, enabling effective visualisation and even potential quantification. Figure 4 provides clear evidence of the even distribution of DIP across the sample surface. 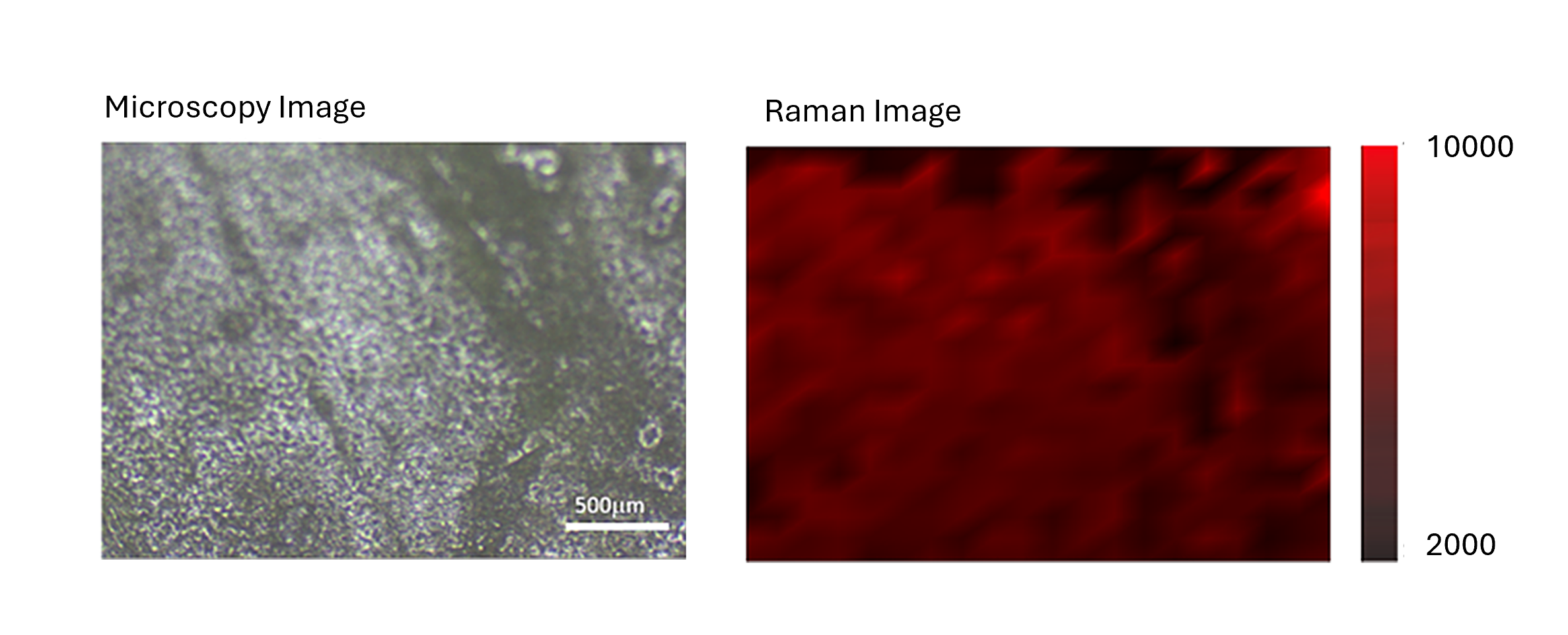
Figure 4. Brightfield image of TPU/10% DIP sample (left panel) and 2D Raman map of the surface based on the intensity of the DIP peak at 1350−1400 cm-1 (right panel).
This Research Highlight demonstrates the importance of using Raman microscopy for the characterisation of 3D-printed materials for bone tissue engineering. An RM5 Raman microscope allowed for clear identification of the TPU polymer and increasing presence of DIP drug in the higher concentrated (TPU/10% DIP) samples. Furthermore, Raman mapping displayed an even distribution of DIP across the TPU/10% DIP 3D-printed sample. Acquiring such characterisation data is crucial in the development of 3D-printed bone/drug implants before being implemented in patients.
The results in this Research Highlight were published in Springer Link Nature within the Journal of Drug Delivery and Translational Research. The full article can be found here: https://doi.org/10.1007/s13346-024-01744-1