The growing demand for lithium, a vital component in electric cars and renewable energy storage solutions, has increased our reliance on energy-intensive hard rock mining.
In hard rock mining, raw lithium-bearing minerals such as α-spodumene (LiAlSi2O6) and petalite (LiAlSi4O10) must be turned into β-spodumene through heating at 1000 °C. This facilitates much easier lithium extraction because the raw minerals have closed monoclinic structures from which it is difficult to leach Li+ cations. On the other hand, β-spodumene has a more open tetragonal structure, Figure 1.
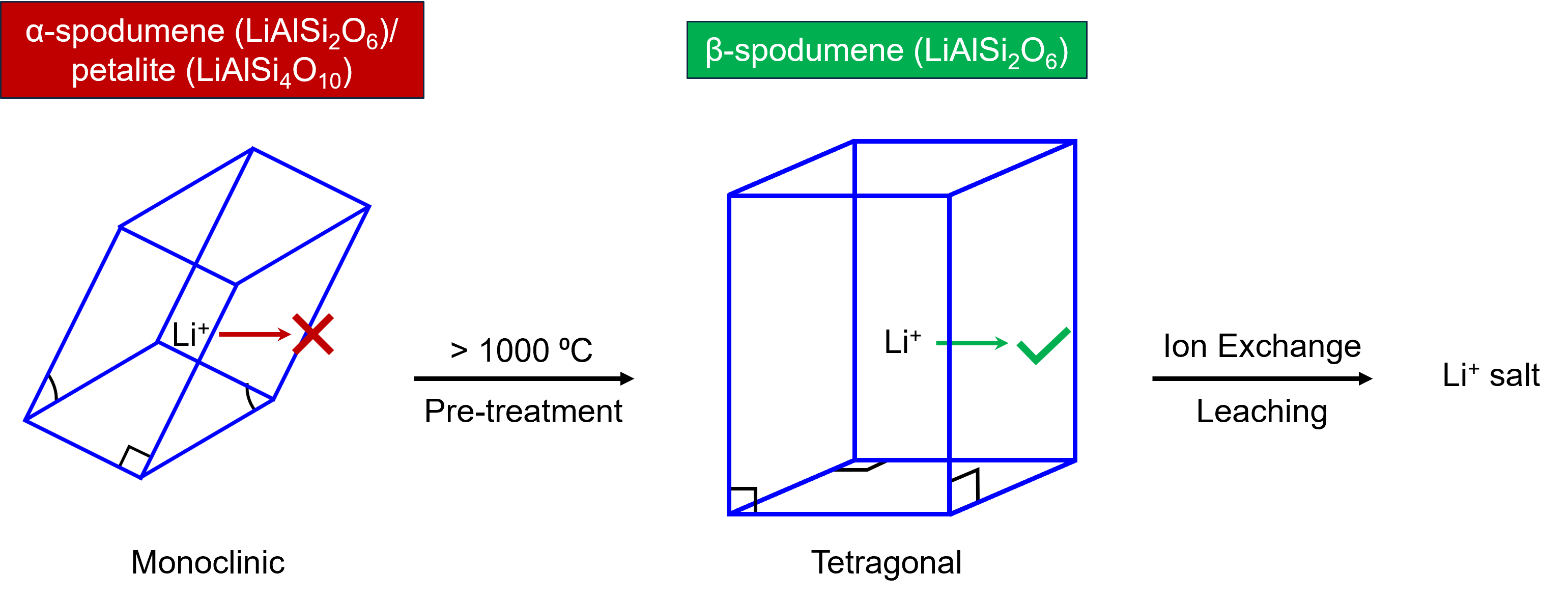
Figure 1. Typical hard-rock mining process for lithium extraction.
To lessen the negative environmental impacts of this process, researchers at CanmetMINING have investigated these crucial phase transitions at lower temperatures. The results of this study were recently published in Crystals.1
Using an Edinburgh Instruments RM5 Confocal Raman Microscope, the researchers found that phase transitions to β-spodumene can occur at 650 °C and 750 °C in α-spodumene and petalite. The Raman microscope was used to locate the inclusions in the minerals that facilitated these phase transitions and identify the formation of β-spodumene through its unique crystal structure. They also confirmed using Raman spectroscopy that sodium doping could promote β-spodumene formation over β-quartz, an unwanted side product. This research could enable increased sustainability in the extraction of lithium.
Raman spectra were collected from α-spodumene and petalite minerals using an RM5 system fitted with a 785 nm laser sourceThe Raman shift x-axis was calibrated periodically to the 520.5 cm-1 band of an internal silicon crystal standard. Each analysis comprised 12 acquisitions of 20 s across a spectral range from 80 cm-1 to 1200 cm-1. Cosmic ray removal was performed in Ramacle® software.

Figure 2. Edinburgh Instruments RM5 Confocal Raman Microscope.
To explore the feasibility of sub-1000 °C phase transitions to β-spodumene, α-spodumene and petalite were heated to various temperatures and then analysed using the RM5. By examining the Raman spectral response from the crystal, the formation of β-spodumene veinlets could be observed within the α-spodumene mineral after heating at 650 °C and doping with sodium, Figure 3. The appearance of a band at 495 cm-1, assigned to the symmetric stretching mode of oxygen bridging two tetrahedral cations (T-O-T), confirmed the α-to-β phase transition. This band is absent in α-spodumene because it has a monoclinic crystal structure.
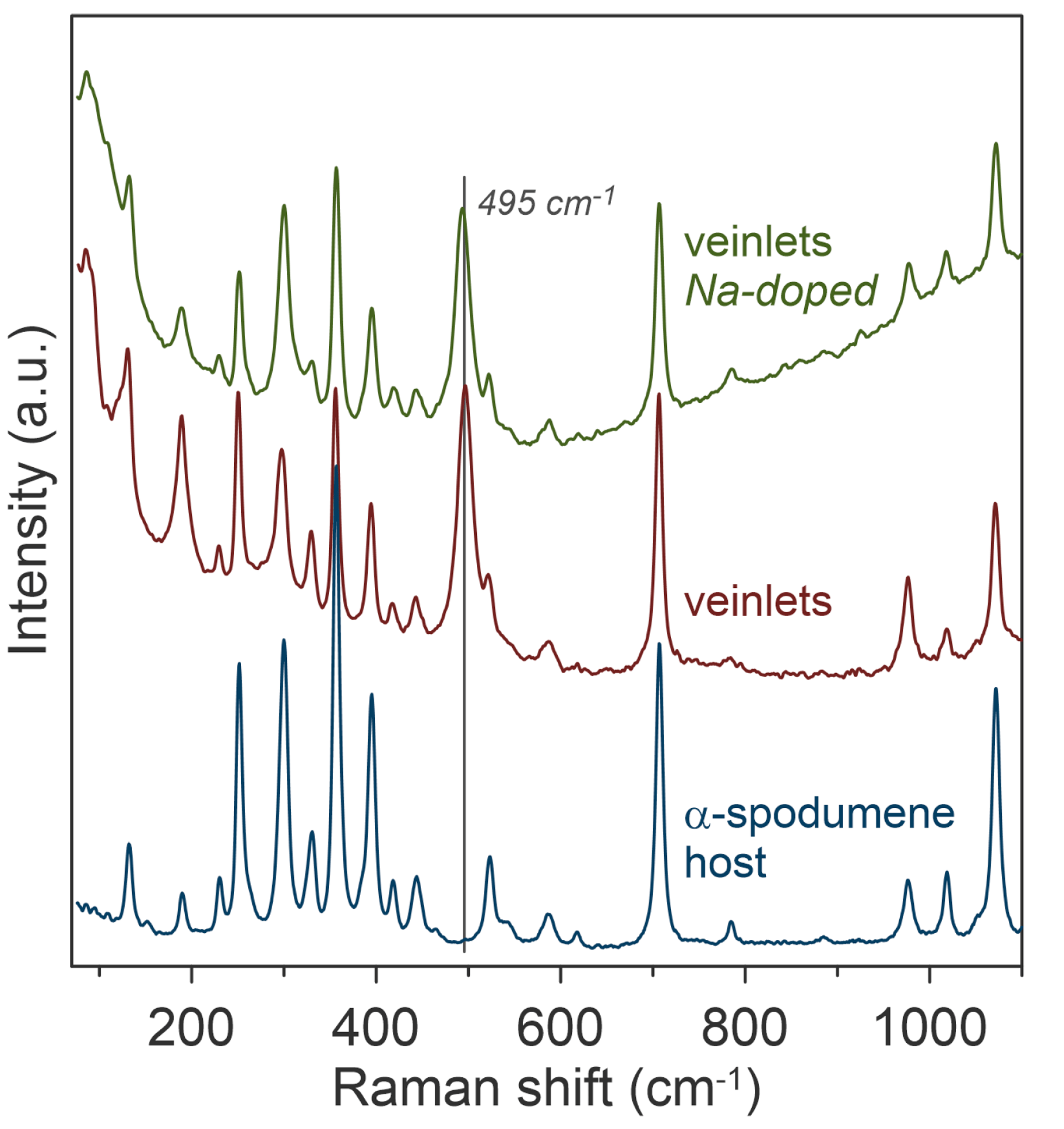
Figure 3. Raman spectroscopic characterisation of the α-β phase transition in spodumene after heat treatment at 650 °C and sodium doping. The image is reprinted from Thibault et al.1, Copyright (2023), with permission from MDPI through an Open Access License.
A 750 °C phase transition from petalite to β-spodumene was also successfully induced. Here, the Raman spectra confirmed the phase transition because the band at 490 cm-1, although present with low intensity in the petalite, became dominant relative to the doublet band at 370 cm-1. This band again represents the symmetric T-O-T stretching band for a tetragonal crystalline lattice, but it is 5 cm-1 lower than β-spodumene formed in the α-spodumene crystal. This is because the Si to Al ratio differs between petalite and α-spodumene.
A low-frequency shoulder was also observed, which could be assigned to the presence of β-quartz. However, this band was no longer present when the experiment was repeated with sodium doping. This suggests that sodium enrichment favours the formation of β-spodumene over β-quartz. These subtle changes in the position and widths of the relevant Raman bands were observable because of the high spectral resolution offered through the combinative effect of using a 785 nm laser with a 1200 gr/mm diffraction grating and closing the spectrograph entrance slit to 50 μm.
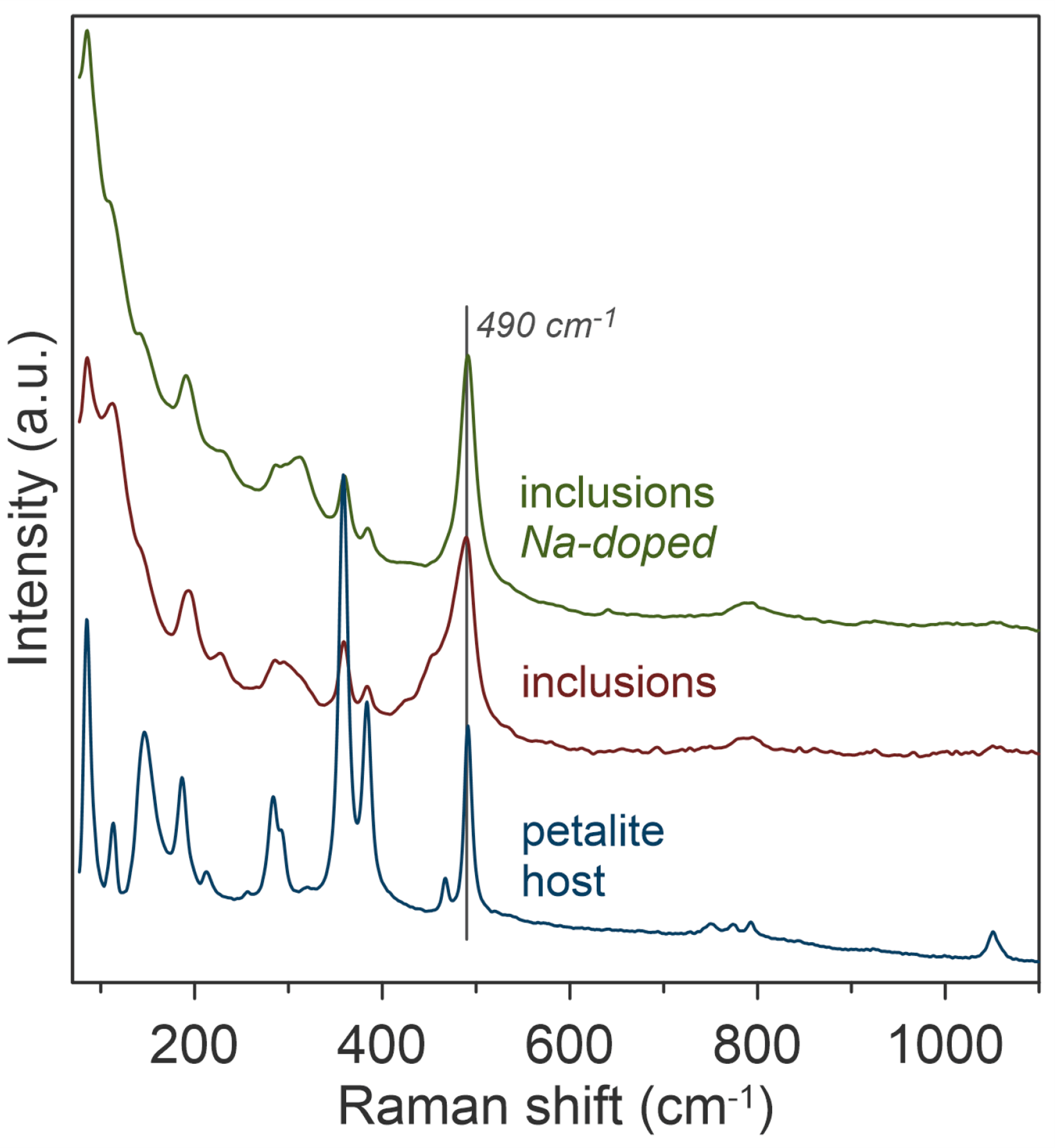
Figure 4. Raman spectroscopic characterisation of the phase transition from petalite to β-spodumene and β-quartz at 750 °C. The image is reprinted from Thibault et al.1, Copyright (2023), with permission from MDPI through an Open Access License.
Since the SiO2:LiAlO2 ratio is twice as large in petalite as it is in α-spodumene, it follows that β-spodumene produced from petalite will be more silica-rich and, therefore, have a lower relative lithium content.
To investigate the impact of an increase in silica on the growth of β-spodumene, synthetic lithium aluminosilicates (LiAlSi₂O₆, LiAlSi₂.9O₇.8, and LiAlSi₄O₁₀) were heated to 1430°C and cooled for crystallisation. Raman spectroscopy identified β-spodumene in LiAlSi₂O₆ and LiAlSi₂.9O₇.8, while only β-quartz was found in LiAlSi₄O₁₀. These spectra are shown in Figure 5. Spectral shifts in the T-O-T stretching band from 495 cm⁻¹ to 492 cm⁻¹ indicated small structural changes in β-spodumene with increasing silica. In β-quartz, the 483 cm⁻¹ band shifted lower with increased silica content, suggesting a transition toward SiO₂-dominant structures.
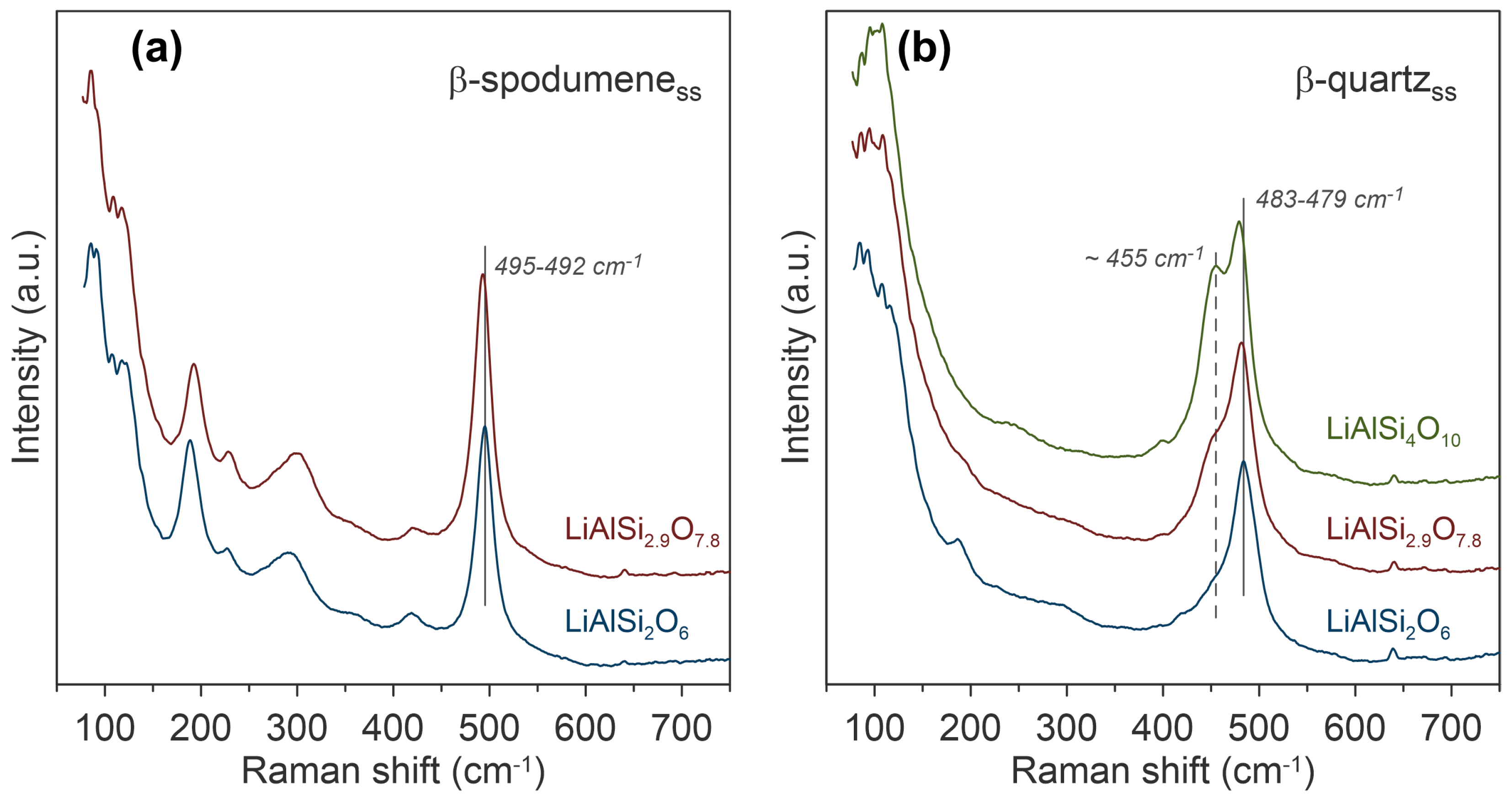
Figure 5. Raman spectroscopic characterisation of the β-spodumene and β-quartz phases in synthetic lithium aluminosilicates crystallised from melts of LiAlSi2O6, LiAlSi2.9O7.8, and LiAlSi4O10. The image is reprinted from Thibault et al.1, Copyright (2023), with permission from MDPI through an Open Access License.
Finally, after treating LiAlSi₂.9O₇.8 with molten NaNO₃ for lithium extraction, Raman analysis confirmed that the β-spodumene and β-quartz structures remained intact, with 65-70% lithium exchanged for sodium, Figure 6.
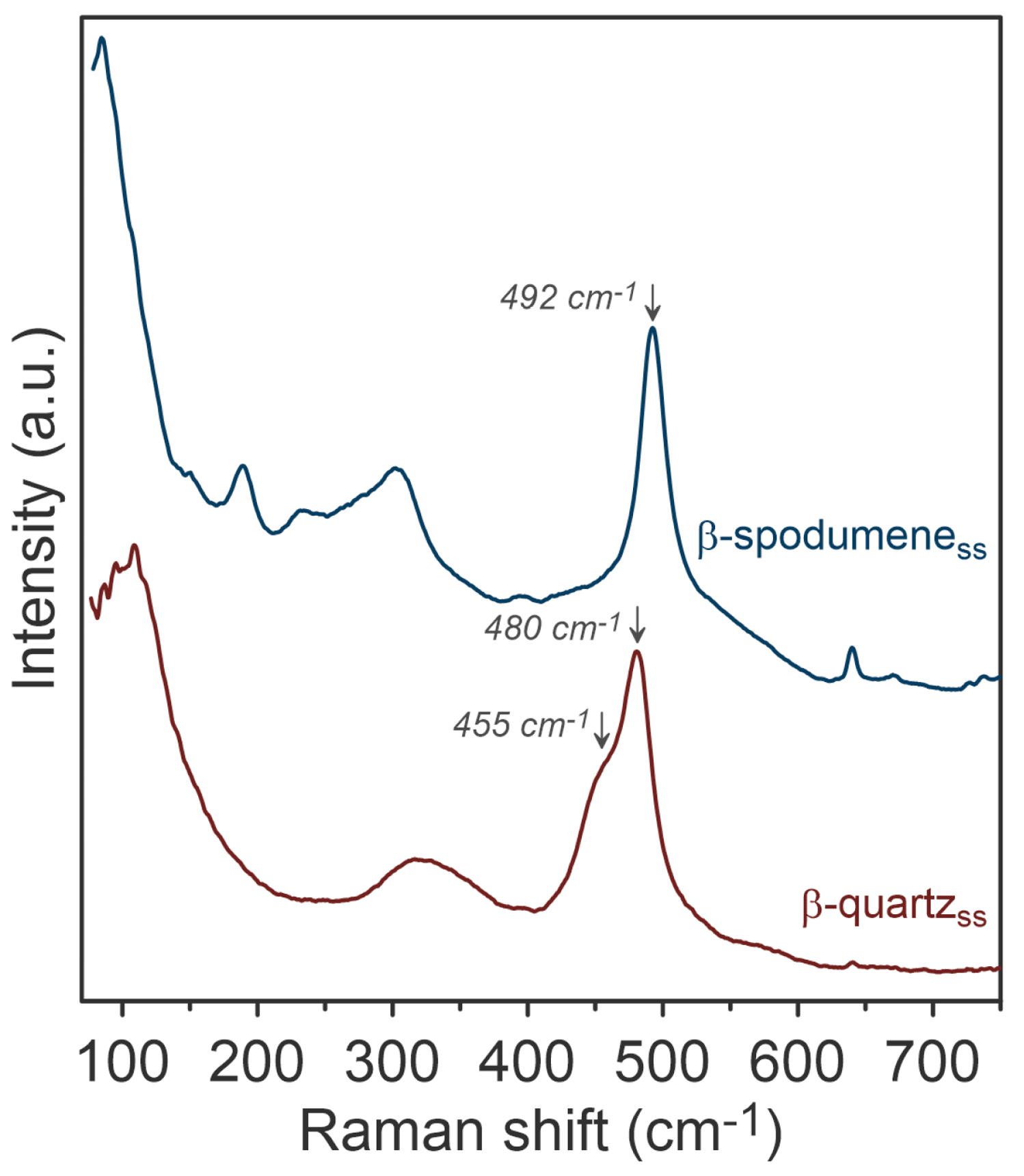
Figure 6. Raman spectroscopic characterisation of the β-spodumene and β-quartz phases in LiAlSi2.9O7.8 fragments after ion exchange in a sodium nitrate molten bath. The image is reprinted from Thibault et al.1, Copyright (2023), with permission from MDPI through an Open Access License.
This Research Highlight shows how can effectively characterise phase transitions caused by low-temperature heat treatment of lithium-bearing minerals. It highlights the efficient differentiation between crystalline structures and confirms sodium doping as an effective method for lithium extraction.
The results shown in this Research Highlight were published in Crystals, 2024. More details can be found here: https://www.mdpi.com/2073-4352/13/8/1182.


