Laser Induced Fluorescence (LIF) is an optical spectroscopic technique where a sample is excited with a laser, and the fluorescence emitted by the sample is subsequently captured by a photodetector. LIF can be understood as a class of fluorescence spectroscopy where the usual lamp excitation is replaced by a laser source. Whilst lasers are now routinely used as excitation sources in photoluminescence spectrometers, Laser Induced Fluorescence was not originally developed for a commercial instrument but as a standalone laser spectroscopy technique.
LIF spectroscopy was first developed by Richard Zare in 1968 for the detection of atoms and molecules in the gas phase.1 Its potential as an analytical technique was quickly realised, as the fluorescence intensity is directly proportional to the concentration of the analyte in the linear power and concentration regime. Laser Induced Fluorescence offers interesting advantages over absorption spectroscopy: zero background, higher selectivity towards the analyte, information about the rotational-vibrational structure of the ground or excited state of the sample, and time-resolved information if using pulsed lasers. In addition, polarisation-dependent measurements are easy to implement since most laser beams are linearly polarised.
One of the first applications of LIF spectroscopy was measuring the temperature of gas-phase samples, and today it is widely used for the analysis of flames.2 The technique soon moved beyond gas samples and into the liquid phase, as it became a detection technique in liquid chromatography and capillary electrophoresis (CE-LIF).3 Nowadays, Laser Induced Fluorescence detection can be found in many contexts, from commercial analytical instruments to advanced microscopy experiments, and it is routinely used in biological and environmental research as well as fundamental spectroscopy studies.
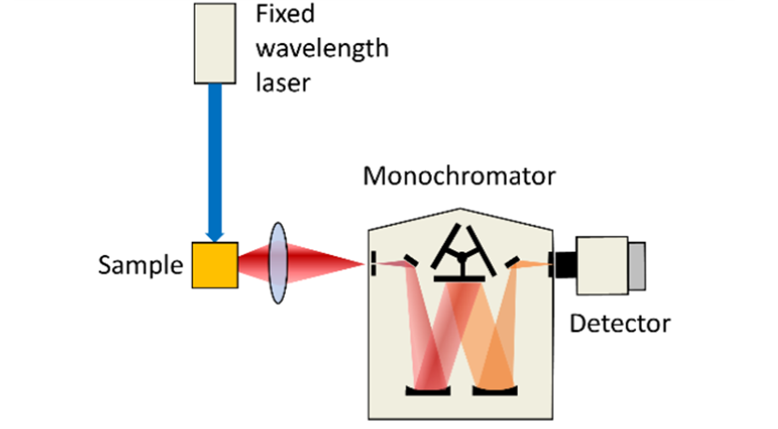
There are different types of Laser Induced Fluorescence spectroscopy depending on the laser and detection system used. It is common to refer to the technique as excitation or emission LIF spectroscopy; Figure 1 illustrates this concept. In the figure a laser is employed to excite molecules from their ground state into an electronically excited state. As the molecules relax back into the ground state, fluorescence is detected by a photomultiplier tube (PMT).
In excitation LIF, the excitation wavelength is varied using a tunable laser which allows one to resolve the vibrational structure of the excited state. In a liquid sample, the molecules will fluoresce from the lowest vibrational level of their excited singlet state, decaying to a series of vibrational levels in the ground state, however the emission spectrum is not resolved by the detection system. A bandpass filter is placed between the sample and PMT to detect all the emission from the sample whilst removing any scattered laser light.
In emission LIF, a fixed pump wavelength is used to excite the sample and the sample’s emission spectrum is analysed utilising a monochromator to select the detection wavelength. The figure shows single-point detection with a PMT, but it is also possible to employ an array detector (CCD or CMOS) to capture the full spectrum in one shot.
Figure 1: Schematic representation of excitation (left) and emission (right) Laser Induced Fluorescence spectroscopy.
It is also possible to classify Laser Induced Fluorescence into continuous wave or time-resolved LIF. Continuous wave (CW) LIF utilises a continuous laser for excitation and is employed when only spectral information is required. In time-resolved LIF, a pulsed laser is used to excite the sample and its emission (either a single wavelength or the full spectrum) is detected as a function of time. This provides valuable time-resolved information such as the lifetimes of chemical intermediates and their associated time-gated spectral evolution. Figure 2 presents an example of time-resolved LIF measured in an Edinburgh Instruments LP980. The ICCD detector in the LP980 enables the acquisition of the full LIF spectrum at varying time delays.
Figure 2: LIF spectra from [Ru(bpy)3]Cl2 acquired in an LP980 with an ICCD detector at different times after the pump pulse (indicated in the graph). λpump = 450 nm, Epump = 10 mJ, gate width = 100 ns.
Edinburgh Instruments incorporates lasers into photoluminescence spectrometers such as the FLS1000 and the FS5, allowing both CW and time-resolved LIF. Pulsed Nd:YAG lasers, which are popular LIF excitation sources, can be integrated with the FLS1000 and the LP980 spectrometers. In particular the LP980 Transient Absorption Spectrometer has been specifically designed for use with these sources, and it can be configured with a holder specific for LIF spectroscopy. In this configuration (Figure 3), the probe lamp is blocked by a shutter controlled by the software as it is not required for LIF, while the pump beam is directed to the sample which sits closer to the collection optic for maximum LIF sensitivity. The LIF configuration in the LP980 Spectrometer maximises the signal detected ensuring the best performance.
Figure 3: Laser Induced Fluorescence configuration of the LP980 spectrometer.