Whilst morphine may be considered an antiquated drug, it is still widely used in medicine and is a metabolite of substances such as heroin and codeine. Accurate detection of controlled substances like morphine is crucial for clinical and forensic applications. Traditional lab-based detection methods such as HPLC and mass spectrometry suffer from high costs, low portability, and the need for specialised training.
Portable, handheld spectrofluorometers combined with selective fluorescent sensors provide a cheap and fast alternative for on-site detection of chemical species. Research-grade instruments such as the FS5 Spectrofluorometer are necessary for the development of these sensors in order to characterise their photoluminescence and determine detection limits. This paper, published in Sensors by researchers from Bournemouth University, reports a novel chemical sensor (7-methoxy-[1,1’-binapthalen]-7-ol, Figure 1a) for morphine that allows sensitive and selective detection of the drug.1 This sensor exhibits a “turn off” response to morphine by binding to the drug through non-covalent interactions, which results in static quenching of the fluorescent sensor (Figure 1b).
![Molecular structure of the sensor, 7-methoxy-[1,1’-binapthalen]-7-ol, (b) binding of the sensor to morphine through Van Der Waals and pi-pi stacking interactions. Reprinted with permission from R. Boroujerdi, et al.](https://edin.becdn.net/wp-content/uploads/2025/10/Molecular-structure-of-the-sensor-and-its-binding-to-morphine.png)
Figure 1: (a) Molecular structure of the sensor, 7-methoxy-[1,1’-binapthalen]-7-ol, (b) binding of the sensor to morphine through Van Der Waals and pi-pi stacking interactions. Reprinted with permission from R. Boroujerdi, et al.
Photoluminescence (PL) spectroscopy measurements were performed on an Edinburgh Instruments FS5 Spectrofluorometer with an extended-range PMT-980 detector which provides spectral coverage up to 950 nm. Solutions of the sensor at a concentration of 100 parts per million (ppm) were tested against standard solutions of morphine and other drugs from 1 parts per billion (ppb) to 100 ppm in methanol.
First the PL properties of the sensor alone were measured. An excitation-emission matrix (EEM) of the sensor in methanol revealed emission and excitation maxima at 369 and 325 nm, respectively (Figure 2).
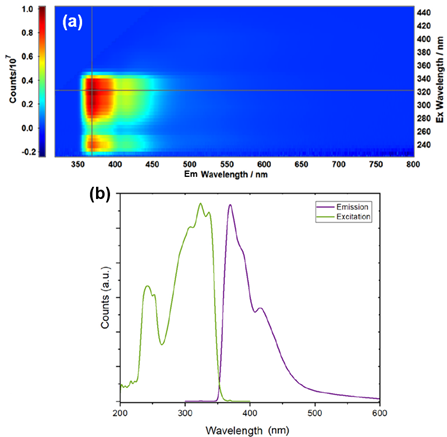
Figure 2: (a) EEM of the sensor in methanol, (b) normalised excitation and emission spectra for the sensor. Reprinted with permission from R. Boroujerdi, et al.
The purity of the synthesised sensor was then approximated using synchronous fluorescence spectroscopy. In this measurement, excitation and emission monochromators are scanned simultaneously with a fixed wavelength offset. This measurement can help to detect the presence of trace impurities which possess distinct fluorescence properties. The sample showed a consistent emission profile across the wavelength range, indicating the product was relatively pure (Figure 3a). Next, the photostability of the sensor was tested by running a series of consecutive measurements over 10 minutes. It was found that the emission of the sensor was highly stable and therefore sufficiently reliable for its application (Figure 3b).
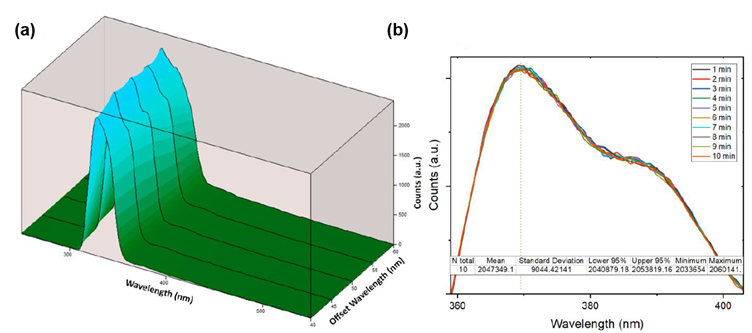
Figure 3: (a) Synchronous fluorescence 3-dimensional plot of the sensor, (b) repeated emission measurements of sensor over 10 mins. Reprinted with permission from R. Boroujerdi, et al.
Next, the PL properties of the sensor with morphine were assessed. First, the emission spectrum of standard solutions of pure morphine in methanol (10 ppb – 50 ppm) was collected. Under excitation at 325 nm, morphine produced a weak emission peak centred at 366 nm (Figure 4a, inset), overlapping with the emission peak of the sensor at 369 nm.
Mixing of the sensor with increasing concentrations of morphine (0 ppb – 40 ppm) produced a reduction in fluorescence signal (Figure 4a). Crucially, the emission of the sensor showed a negative linear relationship with morphine concentration, with a limit of detection as low as 8 ppb (Figure 4b).
For concentrations above 40 ppm, the emission peak of pure morphine acts as the indicator, resulting in a wide dynamic range for this sensing system.
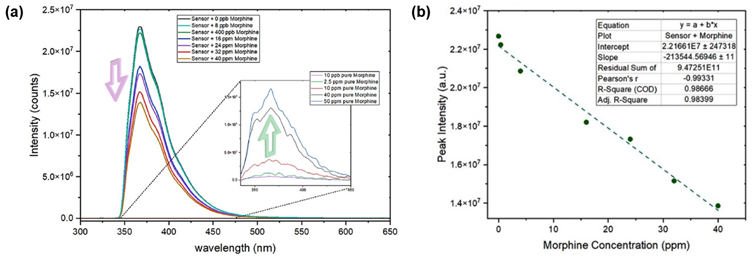
Figure 4: (a) Emission of sensor with increasing concentrations of morphine. Inset: emission spectrum of pure morphine, (b) plot of sensor peak intensity against morphine concentration. Reprinted with permission from R. Boroujerdi, et al.
To assess the selectivity of the sensor for morphine, its output was tested against 20 ppm solutions of other drugs such as ketamine, midazolam, nicotine, and benzylpiperizine (BZP). Fluorescence emission of the sensor + drug (F) was normalised to emission of sensor alone (F’). In the presence of other drugs, the fluorescence intensity of the sensor was either unaffected or increased, with quenching only observed for morphine (Figure 5a).
Next the emission of the sensor in the presence of morphine (20 ppm) mixed with other drugs (20 ppm) was tested. The sensor exhibited showed a decrease in emission, showing that the sensor retains affinity for morphine even in the presence of other substances (Figure 5b).
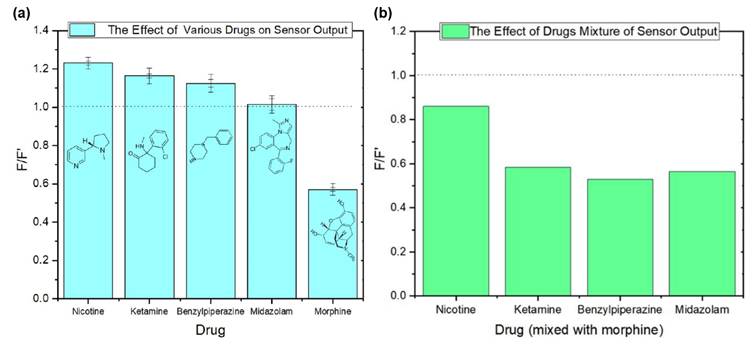
Figure 5: (a) Sensor output in response to morphine and other drugs, (b) sensor output in response to mixtures of morphine with other drugs. Reprinted with permission from R. Boroujerdi, et al.
In this paper, an FS5 Spectrofluorometer was used to develop a fluorescent “turn-off” sensor for morphine. The system showed a wide dynamic range of from 8 ppb – 40 ppm, which outperforms current morphine detection systems such as GC-MS (0.0025 – 2 ppm), colorimetric assays (19.97 – 856 ppb), or piezoelectric sensors (0.25 – 2500 ppb). It is also fast, providing results in under 10 s, is easy to use, and is compatible for use with a portable spectrometer. The high sensitivity of the FS5 allowed for direct detection of the weak PL of morphine, as well as determination of limits of detection down to ppb levels.
This article was published and is available in Sensors.



