If you are someone who uses light in your research, one of the following thoughts may have passed through your mind:
Photoluminescence (PL) spectroscopy provides only so much information to answer these questions as it tells you very little, aside from lifetime, about what is happening between excitation and emission. Or your sample may not emit light at all, and therefore PL can tell you almost nothing.
But there is an alternative technique that is ideal for answering many of these questions: Transient Absorption (TA). This Spectral School will provide a short explanation of the technique, then will dive into some examples of how it can help you understand your sample’s interaction with light.
TA is a “pump-probe” technique that measures the absorption of light by short-lived, “transient” excited state species. It does this by using a light source to excite (“pump”) the sample into a higher energy state, then ”probes” this excited state with a white light source to obtain an absorbance spectrum for the excited states. The difference between the excited state absorbance and the ground state absorbance produces the TA spectrum. By convention, absorbance is measured in units of optical density (OD)1.
The steps involved in performing a TA measurement are:
First, the ground state absorption spectrum is collected. This is achieved by exciting the sample with a white light probe pulse that will promote electrons from its singlet ground state, S0, to a higher excited state, S1 (Figure 1).
1.
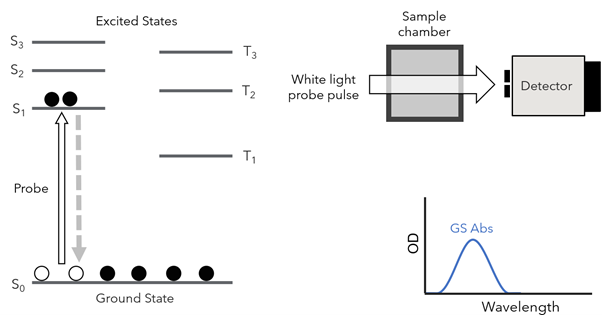
Figure 1: Capture of the ground state absorption spectrum with a probe pulse
Next, the excited state spectrum is collected. A pulsed laser source pumps the sample to a singlet excited state where some electrons may also undergo intersystem crossing (ISC) to a triplet excited state, T1 (Figure 2).
2.
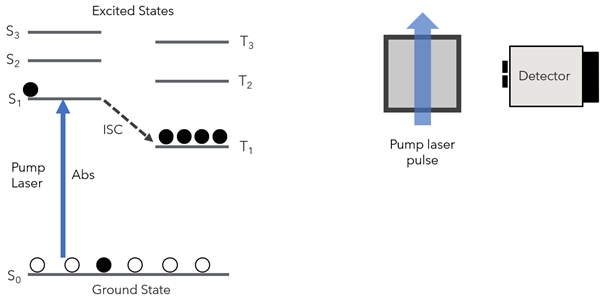
Figure 2: Excitation of the sample with a pump pulse to a singlet excited state, where electrons may undergo ISC to a triplet excited state.
Now that the sample is in the excited state, the probe pulse is used to capture the absorption spectrum by promotion to higher energy excited states (Figure 3). In this example, they are excited to S2 and T2, but they are usually denoted SN and TN.
3.
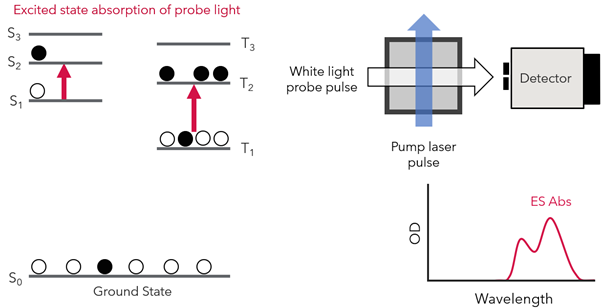
Figure 3: White probe pulse is applied, promoting electrons from first excited states to higher excited states.
4. Once both spectra have been collected, the TA spectrum is calculated from the excited state spectrum minus the ground state spectrum to produce the difference in optical density (ΔOD). By varying the time between the pulse and the probe, ΔOD is measured as a function of time. This can be represented as a series of spectra at different time points, or as a single wavelength decay over time (Figure 4).
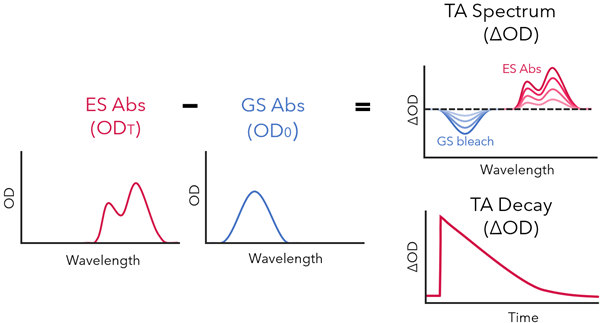
Figure 4: Calculation of the TA “difference spectrum” from the excited state absorption (at time = T) and ground state absorption spectra (at time = 0). Single wavelength measurements may be represented as a function of ΔOD with time.
As the transient species exist on a range of timescales, different instrumentation is required to detect them. For example, singlet excited states tend to have relatively short-excited state lifetimes (picoseconds – nanoseconds), so require ultrafast femtosecond pulsed lasers. Conversely, triplet excited states have relatively long lifetimes (microseconds – seconds) and thus can be measured with nanosecond pulsed lasers. As such, they are regarded as slightly different measurements and are referred to as femtosecond-TA (fs-TA) and nanosecond-TA (ns-TA), respectively.
If molecule AB absorbs a photon and is promoted to its excited state, AB*, several different processes can occur. If it is luminescent, then it can return to its ground state by emitting a photon. However, an excited state may also undergo various non-radiative processes (Figure 5).
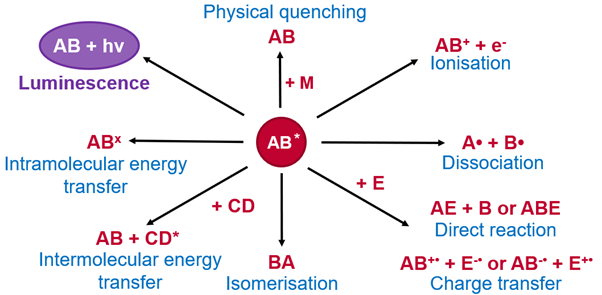
Figure 5: Possible outcomes after molecule AB is promoted to an excited state.
Whilst luminescence can be detected using PL spectroscopy, TA can give you information about all of the other non-radiative processes. This makes it really useful for obtaining mechanistic information about processes involving light. PL and TA should be seen as complementary techniques that fill in the gaps in information that the other cannot provide.
We shall now explore examples from literature of how TA was used to understand reaction mechanisms and perform photophysical characterisation of excited states.
In this example2, researchers prepared a compound to act as a photoinitiator for polymerisation reactions with red light. This is important for applications such as 3D printing and photolithography, the latter of which is used in the semiconductor chip manufacturing.
For their compound to be an efficient photoinitiator, it needed to be able to perform two steps. The first was to undergo ISC to a long-lived triplet excited state. The longer it was in this triplet excited state, the more likely it was for the second step to occur: homolytic cleavage. In this step, a boron-carbon bond would break, forming a highly reactive radical species which would initiate the polymerisation reaction (Figure 6).
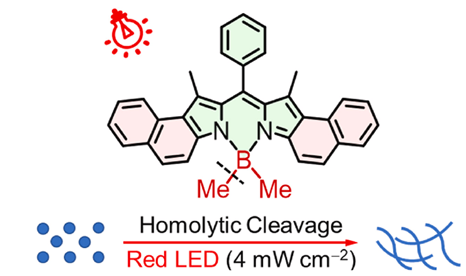
Figure 6: Structure of the photoinitiator. Under red light, the molecule forms a triplet excited state, which then undergoes homolytic cleavage to a radical species which can then start a polymerusation reaction. Figure adapted with permission from Zhang, et al (2025).2
To understand if their compound exhibited these properties, ns-TA and fs-TA were employed.
ns-TA showed the compound possessed an excited state absorption band at 475 nm, which could be attributed to a triplet excited state due to the microsecond timescale. There was also a ground state bleach band at 621 nm which did not fully recover to the baseline. This indicated that the compound had undergone homolytic cleavage under light excitation to form a different compound.
The lifetime of this band was 397 µs, indicating the presence of a triplet state (Figure 7a). This lifetime was reduced to 143 ns when exposed to oxygen, a strong triplet state quencher (Figure 7b). This provided further proof that the mechanism proceeded via ISC to a triplet excited state.
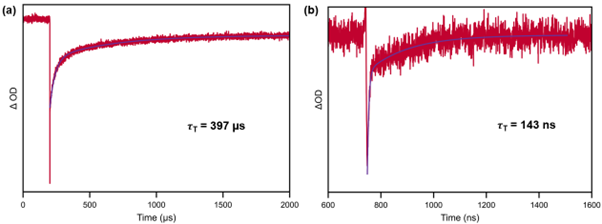
Figure 7: (a) ns-TA measurement of the photoinitator, showing of decay of ground state bleach band at 610 nm under inert atmosphere. (b) ns-TA decay of ground state bleach band at 610 nm in air. Figures replotted with permission from Zhang, et al. (2025).2
fs-TA was used to probe the photoreaction on a faster timescale. It could be observed that the ground state bleach band around 620 nm possessed a biexponential decay corresponding to two distinct processes. One was ISC, and the other was assigned to the decay of radicals generated by photolysis. In contrast, the same molecule with the methyl groups replaced by fluorine showed a monoexponential decay, as the stronger boron-fluorine bond was much less likely to undergo photolysis than the weaker boron-carbon bond.
These results were further confirmed using PL spectroscopy and theoretical calculations, and the researchers were able to demonstrate efficient polymerisation with red light using low amounts of photoinitiator (0.1 mol%).
In this next example3, researchers synthesised a dye based on boron-dipyrromethene (BODIPY) as a photosensitiser for application in photodynamic therapy (PDT). PDT is a medical procedure that utilises light to treat certain types of cancer and specific skin conditions. In this process, light excites a sensitiser to an excited singlet state, which undergoes ISC to the triplet excited state. From here, the photosenstiser is able to convert molecular triplet oxygen (3O2) present in the body to singlet oxygen (1O2). 1O2 is highly toxic and is therefore able to destroy tumour cells.
For a photosensitiser to be effective at generating 1O2, a high triplet quantum yield and a long triplet excited state lifetime are desirable. This means that the sensitiser is able to generate lots of triplet excited states, and the longer its lifetime, the more likely it is to convert 3O2 to 1O2.
Researchers used ns-TA to determine these properties, and were also able to assign photophysical transitions in the mechanism.
The TA spectra of the sensitiser on a microsecond timescale showed the ground state bleach at 610 nm (Figure 8a). This was used to determine its long triplet excited state lifetime as 492 µs (see Figure 8a inset), making it a highly effective PDT agent. Another ns-TA spectrum was taken, but with much higher time resolution to capture the shorter nanosecond timescales. Here, they could observe excited state absorption of three bands, two corresponding to the triplet excited state transitions at 470 nm and 670 nm, as well as a long-lived singlet excited state at 525 nm (Figure 8b). This shows the point in time in which ISC from the singlet to triplet excited state was occurring. They were also able to use ns-TA to calculate the triplet quantum yield, which was found to be 52 %.
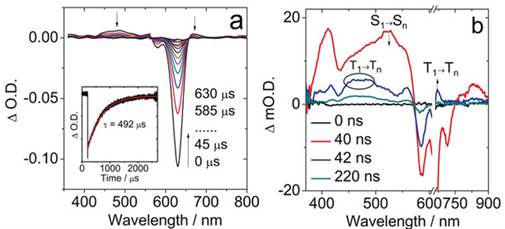
Figure 8: (a) ns-TA spectra of the photosensitiser under excitation 628 nm at microsecond timescales. Inset shows decay of ground state bleach at 610 nm. (b) ns-TA spectra of the photosensitiser under excitation at 628 nm at nanosecond timescales. Figure adapted with permission from Wang. et al. (2020)3
To conclude, ns- and fs-TA are highly effective techniques for understanding the mechanism of how samples interact with light, especially ones that undergo a reaction in their excited state. In the two examples detailed above, TA was used to identify excited states and their lifetimes, key pieces of information for applications in photocatalysis and PDT. They were also able to show that molecules had undergone a reaction in the excited state and that because they possessed triplet excited states, their compounds were sensitive to oxygen in the air.
This kind of information is incredibly useful for various other research areas, including mechanistic photochemistry, LEDs, solar cells, upconversion, photocaging, photoswitches, artificial photosynthesis, quantum dots, optical imaging, and many more.
So if you make samples that interact with light, TA is definitely one technique that could be useful!
At Edinburgh Instruments, we produce the only turnkey ns-TA spectrometer on the market capable of both spectral and kinetic TA measurements – the LP980. This makes TA much more accessible to researchers who don’t want to build their own spectrometer from scratch. In both examples shown above, ns-TA measurements were performed with the LP980.
If you are interested in learning more about the LP980 or any of our products, please get in contact by completing this form!
References
1. Tamai, Y., Murata, Y., Natsuda, S. Ichiro & Sakamoto, Y. How to Interpret Transient Absorption Data?: An Overview of Case Studies for Application to Organic Solar Cells. Adv Energy Mater 14, (2024).
2. Zhang, X. et al. Design and photophysical investigation of a heavy atom-free long-wavelength Type I photoinitiator with efficient photolysis. Dyes and Pigments 240, 112854 (2025).
3. Wang, Z. et al. Elucidation of the Intersystem Crossing Mechanism in a Helical BODIPY for Low-Dose Photodynamic Therapy. Angewandte Chemie – International Edition 59, (2020).