Application Note: Applying Time-Resolved Techniques to a FRET Fluorescence Based Immunoassay
In this application note, we discuss FRET Fluorescence Based Immunoassay after time-resolved techniques have been applied.
Förster resonance energy transfer (FRET) is a phenomenon of non-radiative transfer of the optical excitation from an excited electronic level of one molecule, called the donor, to a resonant electronic level of another molecule, called the acceptor. If the electronic levels of donor (D) and acceptor (A) are in a strong resonance the optical excitation can be up to 10 nm. Efficiency of energy transfer exponentially decays as a sixth power of D-A separation distance. Hence, this phenomenon is used as a “ruler” to measure the separating distance between two molecules or the ascertainment of proximity or spatial coordination of two molecules in solution or on a cell surface. FRET has found massive applications in biology to study protein folding, protein-protein interactions or protein organisation in molecular complexes in solution and on the cellular membrane.
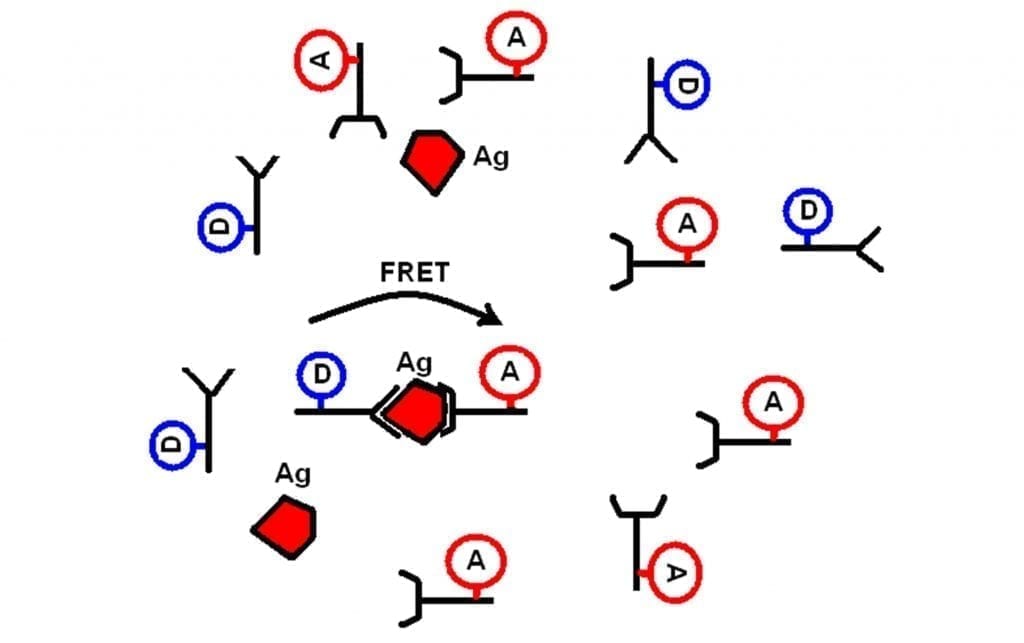
The assay principle is illustrated in the figure below. The assay is based on the specific binding of a “matched” pair of antibodies, AB1 and AB2, to an antigen (Ag) which is the analyte molecule of interest. In the measurements reported here the antigen used was Alpha Fetoprotein (AFP). When both labelled antibodies bind to Ag they produce a ternary complex Ab1-Ag-Ab2. Upon certain experimental conditions when concentration of the donor-labelled antibody is high enough to allow binding of all Ag molecules, but still comparable with Ag concentration, one can resolve a short lifetime component in EuK time-response, the amplitude of which is proportional to the “sandwiches” concentration.
Figure 1: The principle of FRET-based immunoassay
Methods and Materials for FRET Fluorescence
An AFP KRYPTOR kit (B·R·A·H·M·S) was used in this study. The standard protocol for the kit was modified to reduce concentration of the donor-labelled antibody (Ab1-EuK). The reaction was started by mixing 21 μl of Ab1-EuK, 70 μl of Ab2-APC, 49 μl of buffer and 70 μl of Ag solution to give the total volume 210 μl. The final concentration of Ag in the sample varied from 0 to 833 ng/ml. The sample was incubated for 1 hour at 20°C to complete the complex formation reaction. The sample was transferred into a semi-micro fused silica cuvette and time-resolved EuK emission was measured using an FLS980 Fluorescence Spectrometer operating in the multi-channel scaling (MCS) mode. Excitation was provided by a 60 W Xenon microsecond flash lamp with the monochromator set at 310 nm with a 16 nm spectral bandwidth. EuK fluorescence was monitored through the emission monochromator set at 620 nm (16 nm spectral bandwidth). A cooled red sensitive photomultiplier (PMT-900) was used for the gated photon counting (80 ms delay and 10 ms gate width). Emission time-courses were acquired for 30 s.
Download the FRET Fluorescence Based Immunoassay Application Note
Applying Time-Resolved Techniques to a FRET Based Immunoassay
FLS980 Fluorescence Spectrometer
Suitable for both fundamental research and routine laboratory applications, the FLS1000 has replaced our FLS980 Photoluminescence Spectrometer, and sets the standard in steady state and time-resolved photoluminescence spectroscopy. For further information on our FLS1000 Photoluminescence Spectrometer, why not contact one of our sales team at sales@edinst.com.
Stay in Touch
If you have enjoyed this application note, and want to be the first to see all the latest news, applications, and product information from Edinburgh Instruments, then sign-up to our infrequesnt newsletter via the red sign-up button below, and follow us on social media.